Abstract
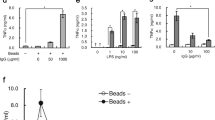
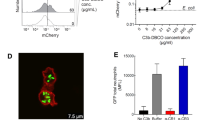
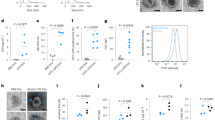
Toll-like receptor 2 (TLR2) initiates inflammation in response to bacterial lipopeptide (BLP). However, the molecular mechanisms enabling the detection of BLP by TLR2 are unknown. Here we investigated the interaction of BLP with human serum proteins and identified vitronectin as a BLP-recognition molecule. Vitronectin and its receptor, integrin β3, were required for BLP-induced TLR2-mediated activation of human monocytes. Furthermore, monocytes from patients with Glanzmann thrombasthenia, which lack integrin β3, were completely unresponsive to BLP. In addition, integrin β3 formed a complex with TLR2 and this complex dissociated after BLP stimulation. Notably, vitronectin and integrin β3 coordinated responses to other TLR2 agonists such as lipoteichoic acid and zymosan. Our findings show that vitronectin and integrin β3 contribute to the initiation of TLR2 responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Janeway, C.A. Jr . & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Schumann, R.R. et al. Structure and function of lipopolysaccharide binding protein. Science 249, 1429–1431 (1990).
Jeannin, P. et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity 22, 551–560 (2005).
Ip, W.K., Takahashi, K., Moore, K.J., Stuart, L.M. & Ezekowitz, R.A. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J. Exp. Med. 205, 169–181 (2008).
He, Y.W. et al. The extracellular matrix protein mindin is a pattern-recognition molecule for microbial pathogens. Nat. Immunol. 5, 88–97 (2004).
Heinzelmann, M. & Bosshart, H. Heparin binds to lipopolysaccharide (LPS)-binding protein, facilitates the transfer of LPS to CD14, and enhances LPS-induced activation of peripheral blood monocytes. J. Immunol. 174, 2280–2287 (2005).
West, A.P., Koblansky, A.A. & Ghosh, S. Recognition and signaling by Toll-like receptors. Annu. Rev. Cell Dev. Biol. 22, 409–437 (2006).
Tobias, P.S. et al. Cross-linking of lipopolysaccharide (LPS) to CD14 on THP-1 cells mediated by LPS-binding protein. J. Immunol. 150, 3011–3021 (1993).
Gioannini, T.L. et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc. Natl. Acad. Sci. USA 101, 4186–4191 (2004).
Braun, V. & Bosch, V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc. Natl. Acad. Sci. USA 69, 970–974 (1972).
Tokuda, H. & Matsuyama, S. Sorting of lipoproteins to the outer membrane in E. coli. Biochim. Biophys. Acta 1693, 5–13 (2004).
Aliprantis, A.O. et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285, 736–739 (1999).
Brightbill, H.D. et al. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285, 732–736 (1999).
Takeuchi, O. et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13, 933–940 (2001).
Takeuchi, O. et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169, 10–14 (2002).
Jin, M.S. et al. Crystal structure of the TLR1–TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082 (2007).
Schroder, N.W. et al. Lipopolysaccharide binding protein binds to triacylated and diacylated lipopeptides and mediates innate immune responses. J. Immunol. 173, 2683–2691 (2004).
Jiang, Z. et al. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6, 565–570 (2005).
Hoebe, K. et al. CD36 is a sensor of diacylglycerides. Nature 433, 523–527 (2005).
Preissner, K.T. Structure and biological role of vitronectin. Annu. Rev. Cell Biol. 7, 275–310 (1991).
Chhatwal, G.S., Preissner, K.T., Muller-Berghaus, G. & Blobel, H. Specific binding of the human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect. Immun. 55, 1878–1883 (1987).
Ginsberg, M.H. et al. Immunochemical and amino-terminal sequence comparison of two cytoadhesins indicates they contain similar or identical beta subunits and distinct alpha subunits. J. Biol. Chem. 262, 5437–5440 (1987).
Suzuki, S. et al. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc. Natl. Acad. Sci. USA 83, 8614–8618 (1986).
Switala-Jelen, K. et al. The biological functions of β3 integrins. Folia Biol. (Praha) 50, 143–152 (2004).
Hynes, R.O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Scibelli, A. et al. Engagement of integrins as a cellular route of invasion by bacterial pathogens. Vet. J. 173, 482–491 (2007).
Nurden, A.T. Glanzmann thrombasthenia. Orphanet J. Rare Dis. 1, 10 (2006).
Gan, Z.R., Gould, R.J., Jacobs, J.W., Friedman, P.A. & Polokoff, M.A. Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus. J. Biol. Chem. 263, 19827–19832 (1988).
Kim, T.W. et al. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J. Exp. Med. 204, 1025–1036 (2007).
Bubeck Wardenburg, J., Williams, W.A. & Missiakas, D. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc. Natl. Acad. Sci. USA 103, 13831–13836 (2006).
Trinchieri, G. & Sher, A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7, 179–190 (2007).
Underwood, P.A., Steele, J.G. & Dalton, B.A. Effects of polystyrene surface chemistry on the biological activity of solid phase fibronectin and vitronectin, analysed with monoclonal antibodies. J. Cell Sci. 104, 793–803 (1993).
Vesy, C.J., Kitchens, R.L., Wolfbauer, G., Albers, J.J. & Munford, R.S. Lipopolysaccharide-binding protein and phospholipid transfer protein release lipopolysaccharides from gram-negative bacterial membranes. Infect. Immun. 68, 2410–2417 (2000).
Heumann, D., Lauener, R. & Ryffel, B. The dual role of LBP and CD14 in response to Gram-negative bacteria or Gram-negative compounds. J. Endotoxin Res. 9, 381–384 (2003).
Hallstrom, T., Trajkovska, E., Forsgren, A. & Riesbeck, K. Haemophilus influenzae surface fibrils contribute to serum resistance by interacting with vitronectin. J. Immunol. 177, 430–436 (2006).
Mukhopadhyay, S., Herre, J., Brown, G.D. & Gordon, S. The potential for Toll-like receptors to collaborate with other innate immune receptors. Immunology 112, 521–530 (2004).
Wooten, R.M. et al. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 160, 5485–5492 (1998).
Wong, K.F., Luk, J.M., Cheng, R.H., Klickstein, L.B. & Fan, S.T. Characterization of two novel LPS-binding sites in leukocyte integrin βA domain. FASEB J. 21, 3231–3239 (2007).
Cuzzola, M. et al. β2 integrins are involved in cytokine responses to whole Gram-positive bacteria. J. Immunol. 164, 5871–5876 (2000).
Kagan, J.C. & Medzhitov, R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125, 943–955 (2006).
Triantafilou, M., Morath, S., Mackie, A., Hartung, T. & Triantafilou, K. Lateral diffusion of Toll-like receptors reveals that they are transiently confined within lipid rafts on the plasma membrane. J. Cell Sci. 117, 4007–4014 (2004).
Wooten, R.M. et al. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168, 348–355 (2002).
Burns, E., Bachrach, G., Shapira, L. & Nussbaum, G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 177, 8296–8300 (2006).
Ylanne, J. et al. Mutation of the cytoplasmic domain of the integrin β3 subunit. Differential effects on cell spreading, recruitment to adhesion plaques, endocytosis, and phagocytosis. J. Biol. Chem. 270, 9550–9557 (1995).
Hodivala-Dilke, K.M. et al. β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 103, 229–238 (1999).
Retta, S.F. et al. Cross talk between β1 and αV integrins: β1 affects β3 mRNA stability. Mol. Biol. Cell 12, 3126–3138 (2001).
Thiede, B. et al. Peptide mass fingerprinting. Methods 35, 237–247 (2005).
Pei, Y. & Tuschl, T. On the art of identifying effective and specific siRNAs. Nat. Methods 3, 670–676 (2006).
Lois, C., Hong, E.J., Pease, S., Brown, E.J. & Baltimore, D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872 (2002).
Acknowledgements
We thank P. Jungblut and M. Schmidt for mass spectrometry; K. Ray and J. Lambers for experimental help; P. Godowski (Genentech) for anti-TLR2; and the EURIT team for siRNA molecules. Supported by the Deutsche Forschungsgemeinschaft (GraKo 1121 to G.G.) and by the European Union Marie Curie Actions (HPMF-CT-2002-01528 to J.L.d.D.).
Author information
Authors and Affiliations
Contributions
G.G. did all experiments unless stated otherwise; K.A.A. and M.B. synthesized the lipopeptides; J.L.d.D. did the BLP precipitation experiments; H.-J.L. was in charge of the patients with Glanzmann thrombasthenia; G.G., A.Z. and J.L.d.D. conceptualized and designed the research and prepared the manuscript; and A.Z. secured the funding.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Methods and Supplementary Tables 1–4 (PDF 1628 kb)
Rights and permissions
About this article
Cite this article
Gerold, G., Abu Ajaj, K., Bienert, M. et al. A Toll-like receptor 2–integrin β3 complex senses bacterial lipopeptides via vitronectin. Nat Immunol 9, 761–768 (2008). https://doi.org/10.1038/ni.1618
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1618