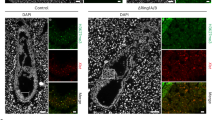
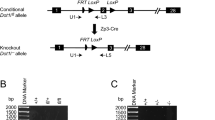
Abstract
In mammals, dosage compensation of X-linked genes is achieved by the transcriptional silencing of one X chromosome in the female (reviewed in ref. 1). This process, called X inactivation, is usually random in the embryo proper. In marsupials and the extra-embryonic region of the mouse, however, X inactivation is imprinted: the paternal X chromosome is preferentially inactivated whereas the maternal X is always active. Having more than one active X chromosome is deleterious to extra-embryonic development in the mouse2. Here we show that the gene eed (embryonic ectoderm development)3,4, a member of the mouse Polycomb group (Pc-G) of genes, is required for primary and secondary trophoblast giant cell development in female embryos. Results from mice carrying a paternally inherited X-linked green fluorescent protein (GFP) transgene implicate eed in the stable maintenance of imprinted X inactivation in extra-embryonic tissues. Based on the recent finding that the Eed protein interacts with histone deacetylases, we suggest that this maintenance activity involves hypoacetylation of the inactivated paternal X chromosome in the extra-embryonic tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Heard, E., Clerc, P. & Avner, P. X-chromosome inactivation in mammals. Annu. Rev. Genet. 31, 571–610 (1997).
Marahrens, Y., Panning, B., Dausman, J., Strauss, W. & Jaenisch, R. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 11, 156–166 (1997).
Faust, C., Lawson, K.A., Schork, N.J., Thiel, B. & Magnuson, T. The Polycomb-group gene eed is required for normal morphogenetic movements during gastrulation in the mouse embryo. Development 125, 4495–4506 (1998).
Schumacher, A., Faust, C. & Magnuson, T. Positional cloning of a global regulator of anterior-posterior patterning in mice. Nature 384, 648 (1996).
Ng, J., Hart, C.M., Morgan, K. & Simon, J.A. A Drosophila ESC-E(Z) protein complex is distinct from other polycomb group complexes and contains covalently modified ESC. Mol. Cell. Biol. 20, 3069–3078 (2000).
Pirrotta, V. Polycombing the genome: PcG, trxG, and chromatin silencing. Cell 93, 333–336 (1998).
Shao, Z. et al. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98, 37–46 (1999).
van der Vlag, J. & Otte, A.P. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nature Genet. 23, 474–478 (1999).
Tie, F., Furuyama, T. & Harte, P.J. The Drosophila Polycomb Group proteins ESC and E(Z) bind directly to each other and co-localize at multiple chromosomal sites. Development 125, 3483–3496 (1998).
Scott, I.C., Anson-Cartwright, L., Riley, P., Reda, D. & Cross, J.C. The HAND1 basic helix-loop-helix transcription factor regulates trophoblast differentiation via multiple mechanisms. Mol. Cell. Biol. 20, 530–541 (2000).
Eggan, K. et al. X-Chromosome inactivation in cloned mouse embryos. Science 290, 1578–1581 (2000).
Hadjantonakis, A.K., Gertsenstein, M., Ikawa, M., Okabe, M. & Nagy, A. Non-invasive sexing of preimplantation stage mammalian embryos. Nature Genet. 19, 220–222 (1998).
Sewalt, R.G. et al. Characterization of interactions between the mammalian polycomb-group proteins Enx1/EZH2 and EED suggests the existence of different mammalian polycomb-group protein complexes. Mol. Cell. Biol. 18, 3586–3595 (1998).
Struhl, G. & Akam, M. Altered distributions of Ultrabithorax transcripts in extra sex combs mutant embryos of Drosophila. EMBO J. 4, 3259–3264 (1985).
Keohane, A.M., Lavender, J.S., O'Neill, L.P. & Turner, B.M. Histone acetylation and X inactivation. Dev. Genet. 22, 65–73 (1998).
Brown, C.J. & Willard, H.F. The human X-inactivation centre is not required for maintenance of X-chromosome inactivation. Nature 368, 154–156 (1994).
Csankovszki, G., Panning, B., Bates, B., Pehrson, J.R. & Jaenisch, R. Conditional deletion of Xist disrupts histone macroH2A localization but not maintenance of X inactivation. Nature Genet. 22, 323–324 (1999).
Gilbert, S.L. & Sharp, P.A. Promoter-specific hypoacetylation of X-inactivated genes. Proc. Natl Acad. Sci. USA 96, 13825–13830 (1999).
Hansen, R.S., Canfield, T.K., Fjeld, A.D. & Gartler, S.M. Role of late replication timing in the silencing of X-linked genes. Hum. Mol. Genet. 5, 1345–1353 (1996).
O'Neill, L.P. et al. A developmental switch in H4 acetylation upstream of Xist plays a role in X chromosome inactivation. EMBO J. 18, 2897–2907 (1999).
Wutz, A. & Jaenisch, R. A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Mol. Cell 5, 695–705 (2000).
Migeon, B.R., Axelman, J. & Beggs, A.H. Effect of ageing on reactivation of the human X-linked HPRT locus. Nature 335, 93–96 (1988).
Sado, T. et al. X inactivation in the mouse embryo deficient for Dnmt1: distinct effect of hypomethylation on imprinted and random X inactivation. Dev. Biol. 225, 294–303 (2000).
Fuks, F., Burgers, W.A., Brehm, A., Hughes-Davies, L. & Kouzarides, T. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nature Genet. 24, 88–91 (2000).
Jones, P.L. et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genet. 19, 187–191 (1998).
Nan, X. et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389 (1998).
Tanaka, M. et al. Parental origin-specific expression of Mash2 is established at the time of implantation with its imprinting mechanism highly resistant to genome-wide demethylation. Mech. Dev. 87, 129–142 (1999).
Rinchik, E.M. & Carpenter, D.A. N-ethyl-N-nitrosourea-induced prenatally lethal mutations define at least two complementation groups within the embryonic ectoderm development (eed) locus in mouse chromosome 7. Mamm. Genome 4, 349–353 (1993).
Acknowledgements
This work was supported by an NIH grant to T.M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Mager, J., Chen, Y. et al. Imprinted X inactivation maintained by a mouse Polycomb group gene. Nat Genet 28, 371–375 (2001). https://doi.org/10.1038/ng574
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng574
This article is cited by
-
Polycomb repressive complexes 1 and 2 are each essential for maintenance of X inactivation in extra-embryonic lineages
Nature Cell Biology (2023)
-
Naïve pluripotent-like characteristics of non-tumorigenic Muse cells isolated from human amniotic membrane
Scientific Reports (2022)
-
Gene regulation in time and space during X-chromosome inactivation
Nature Reviews Molecular Cell Biology (2022)
-
Loss of EZH2-like or SU(VAR)3–9-like proteins causes simultaneous perturbations in H3K27 and H3K9 tri-methylation and associated developmental defects in the fungus Podospora anserina
Epigenetics & Chromatin (2021)
-
Functions and properties of nuclear lncRNAs—from systematically mapping the interactomes of lncRNAs
Journal of Biomedical Science (2020)