Abstract
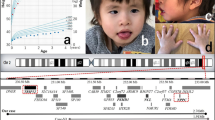
Recently, the application of array-based comparative genomic hybridization (array CGH) has improved rates of detection of chromosomal imbalances in individuals with mental retardation and dysmorphic features1,2,3,4. Here, we describe three individuals with learning disability and a heterozygous deletion at chromosome 17q21.3, detected in each case by array CGH. FISH analysis demonstrated that the deletions occurred as de novo events in each individual and were between 500 kb and 650 kb in size. A recently described 900-kb inversion that suppresses recombination between ancestral H1 and H2 haplotypes5 encompasses the deletion. We show that, in each trio, the parent of origin of the deleted chromosome 17 carries at least one H2 chromosome. This region of 17q21.3 shows complex genomic architecture with well-described low-copy repeats (LCRs)5,6. The orientation of LCRs flanking the deleted segment in inversion heterozygotes is likely to facilitate the generation of this microdeletion by means of non-allelic homologous recombination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Vissers, L.E. et al. Array-based comparative genomic hybridization for the genomewide detection of submicroscopic chromosomal abnormalities. Am. J. Hum. Genet. 73, 1261–1270 (2003).
Shaw-Smith, C. et al. Microarray based comparative genomic hybridisation (array-CGH) detects submicroscopic chromosomal deletions and duplications in patients with learning disability/mental retardation and dysmorphic features. J. Med. Genet. 41, 241–248 (2004).
Cheung, S.W. et al. Development and validation of a CGH microarray for clinical cytogenetic diagnosis. Genet. Med. 7, 422–432 (2005).
Rosenberg, C. et al. Array-CGH detection of micro rearrangements in mentally retarded individuals: clinical significance of imbalances present both in affected children and normal parents. J. Med. Genet. 43, 180–186 (2006).
Stefansson, H. et al. A common inversion under selection in Europeans. Nat. Genet. 37, 129–137 (2005).
Cruts, M. et al. Genomic architecture of human 17q21 linked to frontotemporal dementia uncovers a highly homologous family of low-copy repeats in the tau region. Hum. Mol. Genet. 14, 1753–1762 (2005).
Cassidy, S. & Allanson, B. Management of Genetic Syndromes (Wiley-Liss, Hoboken, New Jersey, 2005).
Flint, J. et al. The detection of subtelomeric chromosomal rearrangements in idiopathic mental retardation. Nat. Genet. 9, 132–140 (1995).
Knight, S.J. et al. Subtle chromosomal rearrangements in children with unexplained mental retardation. Lancet 354, 1676–1681 (1999).
Phelan, M.C. et al. 22q13 deletion syndrome. Am. J. Med. Genet. 101, 91–99 (2001).
Varela, M.C. et al. A 17q21.31 microdeletion encompassing the MAPT gene in a mentally impaired patient. Cytogenet. Genome Res. 114, 89–92 (2006).
Hardy, J. et al. Evidence suggesting that Homo neanderthalensis contributed the H2 MAPT haplotype to Homo sapiens. Biochem. Soc. Trans. 33, 582–585 (2005).
Baker, M. et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 8, 711–715 (1999).
Stankiewicz, P. & Lupski, J.R. Genome architecture, rearrangements and genomic disorders. Trends Genet. 18, 74–82 (2002).
Hurles, M.E. Gene conversion homogenizes the CMT1A paralogous repeats. BMC Genomics 2, 11 (2001).
Lopes, J. et al. Homologous DNA exchanges in humans can be explained by the yeast double-strand break repair model: a study of 17p11.2 rearrangements associated with CMT1A and HNPP. Hum. Mol. Genet. 8, 2285–2292 (1999).
Gimelli, G. et al. Genomic inversions of human chromosome 15q11-q13 in mothers of Angelman syndrome patients with class II (BP2/3) deletions. Hum. Mol. Genet. 12, 849–858 (2003).
Kurotaki, N. et al. Fifty microdeletions among 112 cases of Sotos syndrome: low copy repeats possibly mediate the common deletion. Hum. Mutat. 22, 378–387 (2003).
Visser, R. et al. Identification of a 3.0-kb major recombination hotspot in patients with Sotos syndrome who carry a common 1.9-Mb microdeletion. Am. J. Hum. Genet. 76, 52–67 (2005).
Hutton, M. et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
D'Souza, I. et al. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc. Natl. Acad. Sci. USA 96, 5598–5603 (1999).
Pittman, A.M. et al. Linkage disequilibrium, fine mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J. Med. Genet. 42, 837–846 (2005).
Myers, A.J. et al. The H1c haplotype at the MAPT locus is associated with Alzheimer's disease. Hum. Mol. Genet. 14, 2399–2404 (2005).
Harada, A. et al. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 369, 488–491 (1994).
Ikegami, S., Harada, A. & Hirokawa, N. Muscle weakness, hyperactivity and impairment in fear conditioning in tau-deficient mice. Neurosci. Lett. 279, 129–132 (2000).
Bishop, G.A. & King, J.S. Corticotropin releasing factor in the embryonic mouse cerebellum. Exp. Neurol. 160, 489–499 (1999).
Khalifa, M.M., MacLeod, P.M. & Duncan, A.M. Additional case of de novo interstitial deletion del(17)(q21.3q23) and expansion of the phenotype. Clin. Genet. 44, 258–261 (1993).
Evans, W. et al. The tau H2 haplotype is almost exclusively Caucasian in origin. Neurosci. Lett. 369, 183–185 (2004).
Pittman, A.M. et al. The structure of the tau haplotype in controls and in progressive supranuclear palsy. Hum. Mol. Genet. 13, 1267–1274 (2004).
Acknowledgements
This work was funded by the Wellcome Trust. A.P., R.d.S., and A.J.L. are funded by the Reta Lila Weston Trust for Medical Research. This work is also supported by a grant from the UK Medical Research Council (MRC) to R.d.S. The Brazilian authors are supported by the State of São Paulo Foundation for Research (FAPESP) and the Brazilian National Foundation for Research (CNPq). Arrays were printed by the Microarray Facility of the Wellcome Trust Sanger Institute. The authors thank L. Raymond for use of laboratory facilities, J. Cox for bioinformatics advice and G. Parkin and E. Kerr for technical support. The authors wish to thank the affected individuals and their families for contributing to this research study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
FISH images for selected probes. (PDF 21 kb)
Supplementary Fig. 2
Depiction of possible nonallelic homologous recombination events in inversion heterozygote and homozygote parents. (PDF 20 kb)
Supplementary Table 1
FISH probes used to determine deletion size relative to fully sequenced tiling path clones. (PDF 16 kb)
Supplementary Table 2
Probes used to determine deletion size relative to H1 and H2 haplotypes in patients 1–3. (PDF 48 kb)
Supplementary Table 3
PCR primer sequences for genotyping SNPs and MAPT-repeat-t used in the haplotype analysis in the triads. (PDF 15 kb)
Supplementary Table 4
Genotype results of the SNPs, the H1/H2 insertion/deletion polymorphism and the tetranucleotide MAPT-repeat-t in the three trials. (PDF 30 kb)
Rights and permissions
About this article
Cite this article
Shaw-Smith, C., Pittman, A., Willatt, L. et al. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat Genet 38, 1032–1037 (2006). https://doi.org/10.1038/ng1858
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1858