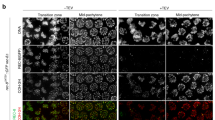
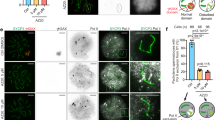
Abstract
In Neurospora, DNA unpaired in meiosis both is silenced and induces silencing of all DNA homologous to it. This process, called meiotic silencing by unpaired DNA, is thought to protect the host genome from invasion by transposable elements. We now show that silencing of unpaired (unsynapsed) chromosome regions also takes place in the mouse during both male and female meiosis. The tumor suppressor protein BRCA1 is implicated in this silencing, mirroring its role in the meiotic silencing of the X and Y chromosomes in normal male meiosis. These findings impact on the interpretation of the relationship between synaptic errors and sterility in mammals and extend our understanding of the biology of Brca1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zickler, D. & Kleckner, N. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 33, 603–754 (1999).
de Boer, P. & de Jong, J.H. Chromosome pairing and fertility in mice. in Fertility and Chromosome Pairing: Recent Studies in Plants and Animals (ed. Gillies, C.B.) 37–76 (CRC Press, Boca Raton, Florida, 1989).
Burgoyne, P.S. & Mahadevaiah, S.K. Unpaired sex chromosomes and gametogenic failure. Chromosomes Today 11, 243–263 (1993).
Miklos, G.L.G. Sex chromosome pairing and male fertility. Cytogenet. Cell Genet. 13, 558–577 (1974).
Burgoyne, P.S. & Baker, T.G. Meiotic pairing and gametogenic failure. in Controlling Events in Meiosis, 38th Symposium of the Society for Experimental Biology (ed. Dickinson, H.G.) 349–362 (The Company of Biologists Ltd, Cambridge, 1984).
Roeder, S. & Bailis, J.M. The pachytene checkpoint. Trends Genet. 16, 395–403 (2000).
Xu, L., Weiner, B.M. & Kleckner, N. Meiotic cells monitor the status of the interhomolog recombination complex. Genes Dev. 11, 106–118 (1997).
Anderson, L.K., Stack, S.M. & Sherman, J.D. Spreading synaptonemal complexes from Zea mays. I. No synaptic adjustment of inversion loops during pachytene. Chromosoma 96, 295–305 (1988).
Moens, P.B. et al. The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA-DNA interactions without reciprocal recombination. J. Cell Sci. 115, 1611–1622 (2002).
Plug, A.W. et al. Changes in protein composition of meiotic nodules during mammalian meiosis. J. Cell Sci. 111, 413–423 (1998).
Ashley, T. An integration of old and new perspectives of mammalian meiotic sterility. in Results and Problems in Cell Differentiation vol. 28 (ed. McElreavey, K.) 131–173 (Springer, Berlin, Heidelberg, 2000).
Keegan, K.S. et al. The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes Dev. 10, 2423–2437 (1996).
Moens, P.B. et al. The association of ATR protein with mouse meiotic chromosome cores. Chromosoma 108, 95–102 (1999).
Scully, R. et al. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 88, 265–275 (1997).
Turner, J.M.A. et al. BRCA1, histone H2AX phosphorylation and male meiotic sex chromosome inactivation. Curr. Biol. (in the press).
Shiu, P.K., Raju, N.B., Zickler, D. & Metzenberg, R.L. Meiotic silencing by unpaired DNA. Cell 107, 905–916 (2001).
Lammers, J.H.M. et al. The gene encoding a major component of synaptonemal complexes of rat is related to X-linked lymphocyte-regulated genes. Mol. Cell. Biol. 14, 1137–1146 (1994).
Lyon, M.F. & Hawker, S.G. Reproductive lifespan in irradiated and unirradiated chromosomally XO mice. Genet. Res. 21, 185–194 (1973).
Burgoyne, P.S. & Baker, T.G. Oocyte depletion in XO mice and their XX sibs from 12 to 200 days post partum. J. Reprod. Fertil. 61, 207–212 (1981).
Burgoyne, P.S. & Baker, T.G. Perinatal oocyte loss in XO mice and its implications for the aetiology of gonadal dysgenesis in XO women. J. Reprod. Fertil. 75, 633–645 (1985).
Speed, R.M. Oocyte development in XO foetuses of man and mouse: the possible role of heterologous X-chromosome pairing in germ cell survival. Chromosoma 94, 115–124 (1986).
Ford, C.E. & Evans, E.P. A reciprocal translocation in the mouse between the X chromosome and a short autosome. Cytogenetics 3, 295–305 (1964).
Odorisio, T., Rodriguez, T.A., Evans, E.P., Clarke, A.R. & Burgoyne, P.S. The meiotic checkpoint monitoring synapsis eliminates spermatocytes via p53-independent apoptosis. Nat. Genet. 18, 257–261 (1998).
Xu, X., Aprelikova, O., Moens, P., Deng, C.X. & Furth, P.A. Impaired meiotic DNA-damage repair and lack of crossing-over during spermatogenesis in BRCA1 full-length isoform deficient mice. Development 130, 2001–2012 (2003).
Xu, X. et al. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat. Genet. 28, 266–271 (2001).
Mahadevaiah, S.K. et al. Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet. 27, 271–276 (2001).
Fernandez-Capetillo, O., Liebe, B., Scherthan, H. & Nussenzweig, A. H2AX regulates meiotic telomere clustering. J. Cell Biol. 163, 15–20 (2003).
Hall, L.L. et al. An ectopic human XIST gene can induce chromosome inactivation in postdifferentiation human HT-1080 cells. Proc. Natl Acad. Sci. USA 99, 8677–8682 (2002).
Huynh, K.D. & Lee, J.T. Inheritance of a pre-inactivated paternal X chromosome in early mouse embryos. Nature 426, 857–862 (2003).
Moore, G.P. DNA-dependent RNA synthesis in fixed cells during spermatogenesis in mouse. Exp. Cell Res. 68, 462–465 (1971).
Handel, M.A. The XY body: a specialized meiotic chromatin domain. Exp. Cell Res. 296, 57–63 (2004).
Turner, J.M.A. et al. Analysis of male meiotic “sex-body” proteins during XY female meiosis provides new insights into their functions. Chromosoma 109, 426–432 (2000).
Wang, P.J. X chromosomes, retrogenes and their role in male reproduction. Trends Endocrinol. Metab. 15, 79–83 (2004).
Bean, C.J., Schaner, C.E. & Kelly, W.G. Meiotic pairing and imprinted X chromatin assembly in Caenorhabditis elegans. Nat. Genet. 36, 100–105 (2004).
Baarends, W.M. et al. Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol. Cell Biol. (in the press).
Mahadevaiah, S.K., Lovell-Badge, R. & Burgoyne, P.S. Tdy-negative XY, XXY and XYY female mice: breeding data and synaptonemal complex analysis. J. Reprod. Fertil. 97, 151–160 (1993).
Peters, A.H., Plug, A.W. & de Boer, P. Meiosis in carriers of heteromorphic bivalents: sex differences and implications for male fertility. Chromosome Res. 5, 313–324 (1997).
Burgoyne, P.S. & Evans, E.P. A high frequency of XO offspring from XPafY* male mice: evidence that the Paf mutation involves an inversion spanning the X PAR boundary. Cytogenet. Cell Genet. 91, 57–61 (2000).
Xu, X. et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat. Genet. 22, 37–43 (1999).
Acknowledgements
We thank C. Heyting and W. Earnshaw for providing antibody reagents; T. Nesterova, L. Hall and J Lawrence for advice on RNA FISH; O. Ojarikre for mouse breeding; and H. Byers for critical reading of the manuscript. J.M.A.T. is an MRC Career Development Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Transcription patterns during male meiosis as revealed by Cot-1 RNA FISH and γH2AX and CREST immunostaining. (PDF 10 kb)
Rights and permissions
About this article
Cite this article
Turner, J., Mahadevaiah, S., Fernandez-Capetillo, O. et al. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 37, 41–47 (2005). https://doi.org/10.1038/ng1484
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1484