Abstract
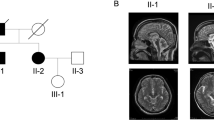
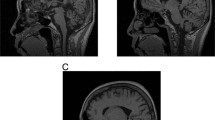
The gene for spinocerebellar ataxia type 2 (SCA2) has been mapped to 12q24.1. A1.1–megabase contig in the candidate region was assembled in P1 artificial chromosome and bacterial artificial chromosome clones. Using this contig, we identified a CAG trinucleotide repeat with CAA interruptions that was expanded in patients with SCA2. In contrast to other unstable trinucleotide repeats, this CAG repeat was not highly polymorphic in normal individuals. In SCA2 patients, the repeat was perfect and expanded to 36–52 repeats. The most common disease allele contained (CAG)37, one of the shortest expansions seen in a CAG expansion syndrome. The repeat occurs in the 5′–coding region of SCA2 which is a member of a novel gene family.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gudmundsson, K. The prevalence and occurrence of some rare neurological diseases in Iceland. Acta. Neurol. Scan. 45, 114–118 (1969).
Orr, H.T. et al. Expansion of an unstable trinucleotide GAG repeat in spinocerebellar ataxia type 1. Nature Genet. 4, 221–226 (1993).
Kawaguchi, Y. et al. CAG expansions in a novel gene for Machado–Joseph disease at chromosome 14q32.1. Nature Genet. 8, 221–228 (1994).
Gispert, S. et al. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA) to chromosome 12q23–24.1. Nature Genet. 4, 295–299 (1993).
Pulst, S.M., Nechiporuk, A. & Starkman, S. Anticipation in spinocerebellar ataxia type 2. Nature Genet. 5, 8–10 (1993).
Flanigan, K. et al. Autosomal dominant spinocerebellar ataxia with sensory axonal neuropathy (SCA4): Clinical description and genetic localization to chromosome 16q22.1. Am. J. Hum. Genet. 59, 392–399 (1996).
Ranum, L.P.W., Schut, L.J., Lundgren, J.K. & Orr, H.T. Spinocerebellar ataxia type 5 in a family descended from the grandparents of president Lincoln maps to chromosome. Nature Genet. 8, 280–284 (1994).
Gouw, L.G. et al. Retinal degeneration characterizes a spinocerebellar ataxia mapping to chromosome 3p. Nature Genet. 10, 89–93 (1995).
Gispert, S. et al. Localization of the candidate gene D–Amino acid oxidase outside the refined l–cM region of spinocerebellar ataxia 2. Am. J. Hum. Genet. 57, 972–975 (1995).
Krauter, K. et al. A second–generation YAC contig map of human chromosome 12. Nature 377, 321–323 (1995).
Nechiporuk, A. et al. Genetic mapping of the spinocerebellar ataxia type 2 gene on human chromosome 12. Neurology 46, 1731–1735 (1996).
Durr, A. et al. Dominant cerebellar ataxia type l linked to chromosome 12q (SCA2: spinocerebellar ataxia type 2). Clin. Neurosci. 3, 12–16 (1995).
The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
La Spada, A.R., Wilson, E.M., Lubahn, D.B., Harding, A.E. & Fischbeck, K.H. Androgen receptor gene mutations in X–linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991).
Koide, R. et al. Unstable expansion of CAG repeat in hereditary dentatorubral–pallidoluysian atrophy (DRPLA). Nature Genet. 6, 9–13 (1994).
Nagafuchi, S. et al. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nature Genet. 6, 14–18 (1994).
Trottier, Y. et al. Polyglutamine expansion as a pathological epitope in Huntington's disease and four dominant cerebellar ataxias. Nature 378, 403–406 (1995).
Loannou, P.A. et al. A new bacteriophage P1–derived vector for the propagation of large human DNA fragments. Nature Genet. 6, 84–89 (1994).
Nechiporuk, T. et al. Identification of three new microsatellite markers in the spiocerebellar ataxia type 2 (SCA2) region and 1.2 Mb physical map. Hum. Genet. 97, 462–467 (1996).
Gacy, A.M., Goellner, G., Juranic, N., Macura, S. & McMurray, C.T. Trinucleotide repeats that expand in human disease form hairpin structures in vitro. Cell 81, 533–540 (1995).
SantaLucia, J., Jr Allawi, H.T. & Seneviratne, P.A. Improved nearest–neighbor parameters for predicting DNA duplex stability. Biochemistry 35, 3555–3562 (1996).
Yamakawa, K. et al. Isolation and characterization of a candidate gene for progressive myoclonus epilepsy on 21q22.3. Hum. Mol. Genet. 4, 709–716 (1995).
Brook, J.D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992).
Fu, Y.H. et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 (1992).
Nelson, D. The fragile X syndromes. Cell Biol. 6, 5–11 (1995).
Goldberg, Y.P. et al. Molecular analysis of new mutations for Huyntington's disease: intermediate alleles and sex of origin effects. Nature Genetics 5, 174–179 (1993).
Myers, R.H. et al. De Novo expansion of a (CAG) repeat in sporadic Huntington's disease. Nature Genet. 5, 168–173 (1993).
Kunst, C.B. & Warren, S.T. Cryptic and polar variation of the fragile X repeat could result in predisposing normal alleles. Cell 77, 853–861 (1994).
Rubinsztein, D.C. et al. Phenotypic characterization of individuals with 30–40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36 to 39 repeats. Am. J. Hum. Genet. 59, 16–22 (1996).
Filla, A. et al. Has spinocerebellar ataxia type 2 a distinct phenotype? Genetic and clinical study of an Italian family. Neurology 45, 793–796 (1995).
McMurray, C.T. Mechanisms of DNA expansion. Chromosoma 104, 2–13 (1995).
Chung, M.Y., Ranum, L., Duvick, L., Servadio, A., Zoghbi, H. & Orr, H.T. Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type 1. Nature Genet. 5, 252–258 (1993).
Burke, J.R. et al. Huntington and DRPLA proteins selectively interact with the enzyme GAPDH. Nature Med. 2, 347–350 (1996).
Ikeda, H. et al. Expanded polyglutamine in the Machado–Joseph disease protein induces cell death in vitro and in vivo. Nature Genet. 13, 196–202 (1996).
Li, X.–J. et al. A huntingtin–associated protein enriched in brain with implications for pathology. Nature 378:, 398–402 (1995).
Cohen, D., Chumakov, I. & Weissenbach, J. A first–generation physical map of the human genome. Nature 366, 698–701 (1993).
Larin, Z. & Lehrach, H. Yeast artificial chromosomes: an alternative approach to the molecular analysis of mouse developmental mutations. Genet. Res. 56, 203–208 (1990).
Korenberg, J.R. & Chen, X.N. Human cDNA mapping using a high resolution R–banding technique and fluorescence In situ–hybridization. Cytogenet. Cell Genet. 69, 196–200 (1995).
Huynh, D., Nechiporuk, T. & Pulst, S.-M. Alternative transcripts in the mouse neurofibromatosis type 2 (NF2) gene are conserved and code for schwannomins with distinct C–terminal domains. Hum. Mol. Genet. 3, 1075–1079 (1994a).
Ralston, M.L. & Jennrich, R.I. DUD, a derivative free algorithm for non–linear least squares. Technometrics 20, 7–14 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pulst, SM., Nechiporuk, A., Nechiporuk, T. et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet 14, 269–276 (1996). https://doi.org/10.1038/ng1196-269
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1196-269