Abstract
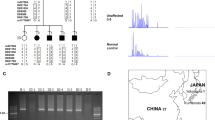
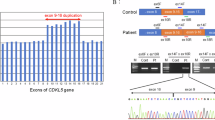
We have identified a novel gene containing CAG repeats and mapped it to chromosome 14q32.1, the genetic locus for Machado-Joseph disease (MJD). In normal individuals the gene contains between 13 and 36 CAG repeats, whereas most of the clinically diagnosed patients and all of the affected members of a family with the clinical and pathological diagnosis of MJD show expansion of the repeat-number (from 68–79). Southern blot analyses and genomic cloning demonstrates the existence of related genes. These results raise the possibility that similar abnormalities in related genes may give rise to diseases similar to MJD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Isselbacher, K.J. et al. in Harrison's Principles of Internal Medicine 13th edn (McGraw-Hill, New York, 1994).
La Spada, A.R., Wilson, E.M., Lubahn, D.B., Harding, A.E. & Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991).
Brook, J.D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68, 799–808 (1992).
Harley, H.G. et al. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature 355, 545–546 (1992).
Buxton, J. et al. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature 355, 547–548 (1992).
Aslanidis, C. et al. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature 355, 548–551 (1992).
Mahadevan, M. et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science 255, 1253–1255 (1992).
Fu, Y.-H. et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255, 1256–1258 (1992).
The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
Orr, H.T. et al. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nature Genet. 4, 221–226 (1993).
Koide, R. et al. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nature Genet. 6, 9–13 (1994).
Nagafuchi, S. et al. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nature Genet. 6, 14–18 (1994).
Fu, Y.-H. et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67, 1047–1058 (1991).
Knight, S.J.L. et al. Trinucleotide repeat amplification and hypermethylation of a CpG island in FRAXE mental retardation. Cell 74, 127–134 (1993).
Duyao, M. et al. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nature Genet. 4, 387–392 (1993).
Snell, R.G. et al. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington's disease. Nature Genet. 4, 393–397 (1993).
Andrew, S.E. et al. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nature Genet. 4, 398–403 (1993).
Gispert, S. et al. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23–24.1. Nature Genet. 4, 295–299 (1993).
Nakano, K.K., Dawson, D.M. & Spence, A. Machado disease. A hereditary ataxia in Portuguse emigrants to Massachusetts. Neurology 22, 49–55 (1972).
Lima, L. & Coutinho, P. Clinical criteria for diagnosis of Machado-Joseph disease: report of a non-Azorean Portuguese family. Neurology 30, 319–322 (1980).
Healton, E.B., Brust, J.C.M., Kerr, D.L., Resor, S. & Penn, A. Presumbly Azorean disease in a presumably non-Portuguese family. Neurology 30, 1084–1089 (1980).
Sakai, T., Ohta, M. & Ishino, H. Joseph disease in a non-Portuguese family. Neurology 33, 74–80 (1983).
Bharucha, N.E., Bharucha, E.P. & Bhabha, S.K. Machado-Joseph-Azorean disease in India. Arch. Neurol. 43, 142–144 (1986).
Suite, N.D.A., Sequeiros, J. & Mckhan, G.M. Machado-Joseph disease in a Sicilian-American family. J. Neurogenet. 3, 177–182 (1986).
Barbeau, A. et al. The natural history of Machado-Joseph disease. An analysis of 138 personally examined cases. Can. J. neurol. Sci. 11, 510–525 (1984).
Rosenberg, R.N. Machado-Joseph disease: An autosomal dominant system degeneration. Mov. Dis. 3, 193–203 (1992).
Takiyama, Y. et al. The gene for Machado-Joseph disease maps to human chromosome 14q. Nature Genet. 4, 300–304 (1993).
Hyslop, P.S.G. et al. Machado-Joseph disease in pedigrees of Azorean descent is linked to chromosome 14. Am. J. hum. Genet. 55, 120–125 (1994).
Krainer, A.R. & Maniatis, T. RNA splicing. in Transcription and splicing (eds Hames, B.D.& Glover, D.M.) 131–206 (IRL press, Oxford, 1988).
Strong, T.V. et al. Widespread expression of the human and rat Huntington's disease gene in brain and nonneural tissues. Nature Genet. 5, 259–265 (1993).
Karp, S.J., Masu, M., Eki, T., Ozawa, K. & Nakanishi, S. Molecular cloning and chromosomal localization of the key subunit of the human N-Methyl-D-aspartate receptor. J. biol. Chem. 268, 3728–3733 (1993).
Kakizuka, A. et al. A mouse cdc25 homologue is differentially and developmentally expressed. Genes Devel. 6, 578–590 (1992).
Maniatis, T., Fritsch, E.F. & Shambrook, J. in Molecular Cloning: a Laboratory Manual2nd edn (Cold Spring Harbor Laboratory Press, New York, 1989).
Taniwaki, M. et al. Detection of 14q32 translations in B-cell malignancies by in situ hybridization with yeast artificial chromosome clones containing the human IgH gene locus. Blood 83, 2962–2969 (1994).
Taniwaki, M. et al. Characterization of two marker chromosomes in a patient with acute nonlymphocytic leukemia by two-color fluorescence in situ hybridization. Cancer Genet. Cytogenet. 70, 99–102 (1993).
Matsuda, F. et al. Structure and physical map of 64 variable segments in the 3′ 0.8-megabase region of the human immunoglobulin heavy-chain locus. Nature Genet. 3, 88–94 (1993).
Vandenplas, S. et al. Blot hybridisation analysis of genomic DNA. J. med. Genet. 21, 164–172 (1984).
Kakizuka, A. et al. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor, PML. Cell 66, 663–674 (1991).
Inoue, J., Kerr, L.D., Kakizuka, A. & Verma, I.M. IκEγ, a70 kd protein identical to the C-terminal half of p110 NF-κB: a new member of the IκB family. Cell 68, 1109–1120 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawaguchi, Y., Okamoto, T., Taniwaki, M. et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet 8, 221–228 (1994). https://doi.org/10.1038/ng1194-221
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1194-221
This article is cited by
-
In planta expression of human polyQ-expanded huntingtin fragment reveals mechanisms to prevent disease-related protein aggregation
Nature Aging (2023)
-
High throughput compound screening in neuronal cells identifies statins as activators of ataxin 3 expression
Scientific Reports (2023)
-
Nucleic acid drug vectors for diagnosis and treatment of brain diseases
Signal Transduction and Targeted Therapy (2023)
-
Association Between Serum Neurofilament Light Chain and Neurochemistry Deficits in Patients with Spinocerebellar Ataxia Type 3
The Cerebellum (2023)
-
The longitudinal progression of MRI changes in pre-ataxic carriers of SCA3/MJD
Journal of Neurology (2023)