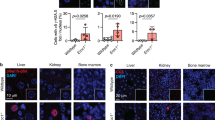
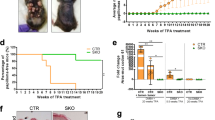
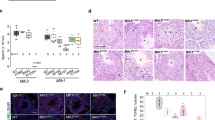
Abstract
Defects in nucleotide excision repair are associated with the human condition xeroderma pigmentosum which predisposes to skin cancer. Mice with defective DNA repair were generated by targeting the excision repair cross complementing gene (ERCC–1) in the embryonic stem cell line, HM–1. Homozygous ERCC–1 mutants were runted at birth and died before weaning with liver failure. Examination of organs revealed polyploidy in perinatal liver, progressing to severe aneuploidy by 3 weeks of age. Elevated p53 levels were detected in liver, brain and kidney, supporting the hypothesised role for p53 as a monitor of DNA damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Friedberg, E.C. in DNA repair (W.H. Freeman, San Francisco, 1985).
Lindahl, T. Instability and decay of the primary structure of DNA. Nature 362, 709–715 (1993).
Weeda, G., Hoeijmakers, J.H.J. & Bootsma, D. Genes controlling nucleotide excision repair in eukaryotic cells. BioEssays 15, 249–258 (1993).
Collins, A.R.S. Mutant rodent cell lines sensitive to UV ionizing radiation and cross linking agents: a comprehensive survey of genetic and biochemical characteristics. Mut. Res. DNA repair 293, 99–118 (1993).
Busch, D. et al. Summary of complementation groups of uv-sensitive CHO cell mutants isolated by large scale screening. Mutagenesis 4, 349–354 (1989).
Cleaver, J.E. & Kraemer, K.H. in The Metabolic Basis of Inherited Disease Vol. 2 (eds Scriver, C.R. et al.) 2949–2971 (McGraw Hill, New York, 1989).
Flejter, W.L., McDaniel, L.D., Johns, D., Friedberg, E.C. & Schultz, R.A. Correction of xeroderma pigmentosum complementation group D mutant cell phenotypes by chromosome and gene transfer: Involvement of the human ERCC2 DNA repair gene. Proc. natn. Acad. Sci. U.S.A. 89, 261–265 (1992).
Weeda, G. et al. A presumed DNA helicase encoded by ERCC-3 is involved in the human repair disorders xeroderma pigmentosum and Cockayne's syndrome. Cell 62, 777–791 (1990).
O'Donovan, A. & Wood, R.D. Identical defects in DNA repair in xeroderma pigmentosum group G and rodent ERCC group 5. Nature 363, 185–188 (1993).
Troelstra, C., Van Gool, A., de Wit, J., Vermeulen, W. & Hoeijmakers, J.H.J. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell 71, 939–953 (1992).
Biggerstaff, M., Szymowski, D.E. & Wood, R.D. Co-correction of the ERCC1, ERCC4 and xeroderma pigmentosum group F DNA repair defects in vitro. EMBO J. 12, 3685–3692 (1993).
Westerveld, A. et al. Molecular cloning of a human DNA repair gene. Nature 310, 425–429 (1984).
van Duin, M. et al. The cloned human DNA excision repair gene ERCC-1 fails to correct xeroderma pigmentosum complementation groups A through I. Mutat. Res. 217, 83–92 (1989).
van Duin, M. et al. Evolution and mutagenesis of the mammalian excision repair gene ERCC-1. Nucl. Acids Res. 16, 5305–5322 (1988).
Biggerstaff, M. & Wood, R.D. Requirement for ERCC-1 and ERCC-3 gene products in DNA excision repair in vitro: complementation using rodent and human cell extracts. J. biol. Chem. 267, 6879–6885 (1992).
Schiestl, R.H. & Prakash, S.A. RAD10, an excision repair gene of Saccharomyces cerevisiae, is involved in the RAD1 pathway of mitotic recombination. Molec. Cell. Biol. 10, 2485–2491 (1990).
Tomkinson, A.E., Bardwell, A.J., Bardwell, L., Tappe, N.J. & Friedberg, E.C. Yeast DNA repair and recombination proteins Rad1 and Rad10 constitute a single-stranded-DNA endonuclease. Nature 362, 860–862 (1993).
Belt, P.B.G.M., Van Oosterwijk, M.F., Odijk, H., Hoeijmakers, J.H.J. & Backendorf, C. Induction of a mutant phenotype in human repair proficient cells after overexpression of a mutated human DNA repair gene. Nucl. Acids Res. 19, 5633–5637 (1991).
Gulyas, K.D. & Donahue, T.F. SSL2, a suppressor of a stem-loop mutation in the HIS4 leader encodes the yeast homolog of human ERCC-3. Cell 69, 1031–1042 (1992).
Schaeffer, L.S. et al. DNA repair helicase: a component of BTF2(TFIIH) basic transcription factor. Science 260, 58–63 (1993).
Mitchell, D.L. & Hartman, P.S. The regulation of DNA repair during development. BioEssays 12, 74–79 (1990).
Vogelstein, B. & Kinzler, K.W. p53 function and dysfunction. Cell 70, 523–526 (1992).
Kastan, M.B., Onyekwere, O., Sidransky, D., Vogelstein, B. & Craig, R.W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51, 6304–6311 (1991).
Lane, D.P. p53, guardian of the genome. Nature 358, 15–16 (1992).
Selfridge, J., Pow, A.M., McWhir, J., Magin, T.M. & Melton, D.W. Gene targeting using a mouse HPRT minigene/HPRT-deficient embryonic stem cell system: Inactivation of the mouse ERCC-1 gene. Som. Cell molec. Genet. 18, 325–336 (1992).
Regan, J.D. et al. Cyclobutane—pyrimidine dimer excision in UV-sensitive CHO mutants and the effect of the ERCC2 repair gene. Mut. Res. 235, 157–160 (1990).
Squires, S., Johnson, R.T. & Collins, A.R.S. Initial rates of DNA incision in UV-irradiated human cells. Differences between normal, XP and tumour cells. Mut Res. 95, 389–404 (1982).
van Duin, M. et al. Genomic characterisation of the human DNA excision repair gene ERCC-1. Nucl. Acids Res. 15, 9195–9213 (1987).
Midgley, C.A. et al. Analysis of p53 expression in human tumours: an antibody raised against human p53 expressed in Escherichia coli. J. cell Sci. 101, 183–189 (1992).
Sancar, A. & Sancar, G.B. DNA repair enzymes. A. Rev. Biochem. 57, 29–67 (1988).
Voigt, J.M., van Houten, B., Sancar, A. & Topal, M.D. Repair of O6-methylguanine by abc excinuclease of E. coli in vitro. J. biol. Chem. 264, 5172–5176 (1989).
Yoke, W.K., Wallace, S.S. & van Houten, B. uvr ABC nuclease complex repairs thymine glycol, an oxidative DNA base damage. Mut. Res. 235, 147–156 (1990).
Olanow, C.W. An introduction to the free radical hypothesis in Parkinson's disease. Ann. Neurol. 32, S2–S9 (1992).
Harrison, D.J., Kharbanda, R., Cunningham, D.S., McLellan, L.I. & Hayes, J.D. Distribution of glutathione S-transferase isoenzymes in human kidney. J. clin. Pathol. 42, 624– 628 (1989).
Epstein, C.J. & Gatens, E.A. Nuclear ploidy in mammalian parenchymal liver cells. Nature 214, 1050–1051 (1967).
Pinkus, R., Bergelson, S. & Daniel, V. Phenobarbitol induction of AP1 binding activity mediates activation of glutathione S-transferase and quinone reductase gene expression. Biochem. J. 290, 637–640 (1993).
Clarke, A.R. et al. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362, 849–852 (1993).
Smith, A.G. et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336, 688–690 (1988).
Bradley, A., Evans, M., Kaufman, M.H. & Robertson, E. Formation of germline chimaeras from embryo-derived teratocarcinoma cells. Nature 309, 255–258 (1984).
Thompson, S., Clarke, A.R., Pow, A.M., Hooper, M.L. & Melton, D.W. Germline transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell 56, 313–321 (1989).
Jenkins, J.R., Rudge, K., Redmond, S. & Wadeevans, A. Cloning and expression analysis of full length mouse cDNA sequences encoding the transformation associated protein p53. Nucl. Acids Res. 12, 5609–5626 (1984).
Minty, A.J. et al. Construction and characterisation of a recombinant plasmid molecule containing a complementary DNA transcript of mouse α actin mRNA. J. biol. Chem. 256, 1008–1014 (1981).
Ormerod, M.G. . in Flow Cytometry (ed. Ormerod, M.G.) 69–86 (IRL Press, Oxford, 1990).
Brugal, G. et al. HOME: Highly optimized microscope environment. Cytometry 13, 109–116 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McWhir, J., Selfridge, J., Harrison, D. et al. Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning. Nat Genet 5, 217–224 (1993). https://doi.org/10.1038/ng1193-217
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1193-217
This article is cited by
-
Pathological consequences of DNA damage in the kidney
Nature Reviews Nephrology (2023)
-
Fanconi anemia DNA crosslink repair factors protect against LINE-1 retrotransposition during mouse development
Nature Structural & Molecular Biology (2023)
-
Mitochondrial phosphatase PGAM5 modulates cellular senescence by regulating mitochondrial dynamics
Nature Communications (2020)
-
DNA cross-link repair safeguards genomic stability during premeiotic germ cell development
Nature Genetics (2019)
-
ERCC1–XPF cooperates with CTCF and cohesin to facilitate the developmental silencing of imprinted genes
Nature Cell Biology (2017)