Abstract
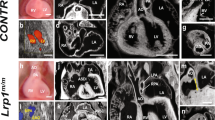
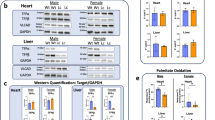
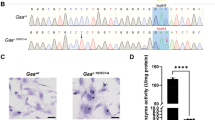
The cerebro-hepato-renal syndrome of Zellweger is a fatal inherited disease caused by deficient import of peroxisomal matrix proteins. The pathogenic mechanisms leading to extreme hypotonia, severe mental retardation and early death are unknown. We generated a Zellweger animal model through inactivation of the murine Pxr1 gene (formally known as Pex5) that encodes the import receptor for most peroxisomal matrix proteins. Pxr1−/− mice lacked morphologically identifiable peroxisomes and exhibited the typical biochemical abnormalities of Zellweger patients. They displayed intrauterine growth retardation, were severely hypotonic at birth and died within 72 hours. Analysis of the neocortex revealed impaired neuronal migration and maturation and extensive apoptotic death of neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Reddy, J.K. & Mannaerts, G.P. Peroxisomal lipid metabolism. Annu. Rev. Nutr. 14, 343–370 (1994).
Mannaerts, G.P. & van Veldhoven, P.P. Metabolic role of mammalian peroxisomes. in Peroxisomes: Biology and Importance in Toxicology and Medicine (eds Gibson, G. & Lake, B.) 19–62 (Taylor & Francis, London, 1993).
Krisans, S.K. Cell compartimentalization of cholesterol biosynthesis, in Peroxisomes: Biology and Role in Toxicology and Disease (eds Reddy, J. K., Suga, T., Mannaerts, G.P., Lazarow, P.B. & Subramani, S.) 142–164 (New York Academy of Sciences, New York, 1996).
Subramani, S. Protein import into peroxisomes and biogenesis of the organelle. Annu. Rev. Cell Biol. 9, 445–478 (1993).
Subramani, S. Protein translocation into peroxisomes. J. Biol. Chem. 271, 32483–32486 (1996).
Distel, B. et al. A unified nomenclature for peroxisome biogenesis factors. J. Cell Biol. 135, 1–3 (1996).
Braverman, N., Dodt, G., Gould, S.J. & Valle, D. Disorders of peroxisome biogenesis. Hum. Mol. Genet. 4, 1791–1798 (1995).
Wanders, R.J.A., Barth, P.G., Schutgens, R.B.H. & Tager, J.M. Peroxisomal disorders, in Peroxisomes: Biology and Importance in Toxicology and Medicine (eds Gibson, G. & Lake, B.) 63–98 (Taylor & Francis, London, 1993).
Lazarow, P. & Moser, H. Disorders of peroxisomal biogenesis, in The Metabolic Basis of Inherited Disease (eds Beaudet, A. L., Scriver, C.R., Sly, W.S. & Valle, D.) 1479–1509 (McGraw-Hill, New York, 1989).
Heymans, H.S.A., Schutgens, R.B.H., Tan, R., van den Bosch, H. & Borst, P. Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome). Nature 306, 69–70 (1983).
Powers, J.M. The pathology of peroxisomal disorders with pathogenetic considerations. J. Neuropathol. Exp. Neurol. 54, 710–719 (1995).
Evrard, P., Caviness, V.S., Prats-Vinas, J. & Lyon, G. The mechanism of arrest of neuronal migration in the Zellweger malformation: an hypothesis based upon cytoarchitectonic analysis. Acta Neuropathol. 41, 109–117 (1978).
Dodt, G. et al. Mutations in the PTS1 receptor gene, PXR1, define complementation group 2 of the peroxisome biogenesis disorders. Nature Genet. 9, 115–125 (1995).
Fransen, M. et al. Identification and characterization of the putative human peroxisomal C-terminal targeting signal import receptor. J. Biol. Chem. 270, 7731–7736 (1995).
Wiemer, E.A.C. et al. Human peroxisomal targeting signal-1 receptor restores peroxisomal protein import in cells from patients with fatal peroxisomal disorders. J. Cell Biol. 130, 51–65 (1995).
Brocard, C., Kragler, F., Simon, M.M., Schuster, T. & Hartig, A. The tetratricopeptide repeat-domain of the PAS10 protein of Saccharomyces cerevisiae is essential for binding the peroxisomal targeting signal-SKL. Biochem. Biophys. Res. Commun. 204, 1016–1022 (1994).
Baker, J., Liu, J., Robertson, E.J. & Efstratiadis, A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75, 73–82 (1993).
Powell-Braxton, L. et al. IGF-1 is required for normal embryonic growth in mice. Genes Dev. 7, 2609–2617 (1993).
Goldstein, J.L. & Brown, M.S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Carlberg, M. et al. Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface. J. Biol. Chem. 271, 17453–17462 (1996).
Fahimi, H.D. Cytochemical localization of peroxidatic activity of catalase in rat hepatic microbodies (peroxisomes). J. Cell Biol. 43, 275–288 (1969).
Wiemer, E.A.C. et al. Presence of peroxisomal membrane proteins in liver and fibroblasts from patients with the Zellweger syndrome and related disorders: evidence for the existence of peroxisomal ghosts. Eur. J. Cell Biol. 50, 407–417 (1989).
Espeel, M. et al. Immunolocalization of a 43 kDa peroxisomal membrane protein in the liver of patients with generalized peroxisomal disorders. Eur. J. Cell Biol. 67, 319–327 (1995).
Johnson, A.B., Schaumburg, H.H. & Powers, J.M. Histochemical characteristics of the striated inclusions of adrenoleukodystrophy. J. Histochem. Cytochem. 24, 725–730 (1976).
Powers, J.M., Tummons, R.C., Caviness, V.S., Moser, A.B. & Moser, H.W. Structural and chemical alterations in the cerebral maldevelopment of fetal cerebro-hepatorenal (Zellweger) syndrome. J. Neuropathol. Exp. Neurol. 48, 270–289 (1989).
Gressens, P. et al. Early neurogenesis and teratogenesis in whole mouse embryo cultures: histochemical, immunocytological and ultrastructural study of the premigratory neuronal-glial units in normal mouse embryo and in mouse embryos influenced by cocaine and retinoic acid. J. Neuropathol. Exp. Neurol. 51, 206–219 (1992).
Agamanolis, D.P., Robinson, H.B.J. & Timmons, G.D. Cerebro-hepato-renal syndrome; report of a case with histochemical and ultrastructural observations. J. Neuropathol. Exp. Neurol. 35, 226–246 (1976).
Roels, F. et al. Cell and tissue heterogeneity in peroxisomal patients, in Functions and Biogenesis of Peroxisomes in Relation to Human Disease (eds Wanders, R.J.A., Schutgens, R.B.H. & Tabak, H.F.) 271–294 (North-Holland, Amsterdam, 1995).
Braverman, N. et al. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nature Genet. 15, 369–376 (1997).
Motley, A.M. et al. Rhizomelic chondrodysplasia punctata is a peroxisomal protein targeting disease caused by a non-functional PTS2 receptor. Nature Genet. 15, 377–380 (1997).
Purdue, P.E., Zhang, J.W., Skoneczny, M. & Lazarow, P.B. Rhizomelic chondrodysplasia punctata is caused by deficiency of human PEX7, a homologue of the yeast PTS2 receptor. Nature Genet. 15, 381–384 (1997).
Motley, A., Hettema, E., Distel, B. & Tabak, H. Differential protein import deficiencies in human peroxisome assembly disorders. J. Cell Biol. 125, 755–767 (1994).
Slawecki, M.L. et al. Identification of three distinct peroxisomal protein import defects in patients with peroxisome biogenesis disorders. J. Cell Sci. 108, 1817–1829 (1995).
Goldfisher, S. et al. Peroxisomal and mitochondrial defects in the cerebro-hepatorenal syndrome. Science 182, 62–64 (1973).
Tybulewisz, V.L., Crawford, C.E., Jackson, P.K., Bronson, R.T. & Mulligan, R.C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153–1163 (1991).
Carmeliet, P. et al. Plasminogen activator inhibitor–1 gene-deficient mice: I. Generation by homologous recombination and characterization. J. Clin. Invest. 92, 2746–2755 (1993).
van Veldhoven, P.P. Activity measurements of acyl-CoA oxidases in human liver. J. Inherited Metab. Dis. 18, 125–134 (1995).
Jones, K.M. & Hajra, A.K. Assay of dihydroxyacetone phosphate acyltransferase with 32P-labeled substrate [letter]. Clin. Chem. 40, 946–947 (1994).
Vanhove, G. et al. Mitochondrial and peroxisomal β-oxidation of the branched chain fatty acid 2-methylpalmitate in rat liver. J. Biol. Chem. 266, 24670–24675 (1991).
van Veldhoven, P. & Bell, R.M. Effect of harvesting methods, growth conditions and growth phase on diacylglycerol levels in cultured human adherent cells. Biochim. Biophys. Acta 959, 185–196 (1988).
Kaluzny, M.A., Duncan, L.A., Merritt, M.V. & Epps, D.E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J. Lipid Res. 26, 135–140 (1985).
Blank, M.L., Cress, E.A., Piantadosi, C. & Snyder, F. A method for the quantitative determination of glycerolipids containing o-alkyl and o-alk-1-enyl moieties. Biochim. Biophys. Acta 380, 208–218 (1975).
Moser, H.W. & Moser, A.B. Measurements of saturated very long chain fatty acids in plasma, in Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual (ed Hommes, F. A.) 177–191 (Wiley-Liss, New York, 1991).
Baumgart, E., Völkl, A., Hashimoto, T. & Fahimi, H.D. Biogenesis of peroxisomes: immunocytochemical investigation of peroxisomal membrane proteins in proliferating rat liver peroxisomes and in catalase-negative membrane loops. J. Cell Biol. 108, 2221–2231 (1989).
Fahimi, H.D. & Baumgart, E., Peroxisomes. in Electron Microscopic Cytochemistry and Immunocytochemistry in Biomedicine (eds Ogawa, K. & Barka, T.) 491–504 (CRC Press, Boca Raton, Florida, 1996).
Baumgart, E. Morphology of peroxisomes in light and electron microscopy, in Peroxisomes (eds. Latruffe, N. & Bugaut, M.) 37–57 (Springer-Verlag, Heidelberg, 1994).
Fransen, M., Brees, C., van Veldhoven, P.P. & Mannaerts, G.P. The visualization of peroxisomal proteins containing a C-terminal targeting sequence on Western blot by using the biotinylated PTS1-receptor. Anal. Biochem. 242, 26–30 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baes, M., Gressens, P., Baumgart, E. et al. A mouse model for Zellweger syndrome. Nat Genet 17, 49–57 (1997). https://doi.org/10.1038/ng0997-49
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0997-49
This article is cited by
-
Loss of pex5 sensitizes zebrafish to fasting due to deregulated mitochondria, mTOR, and autophagy
Cellular and Molecular Life Sciences (2023)
-
PEX5 regulates autophagy via the mTORC1-TFEB axis during starvation
Experimental & Molecular Medicine (2018)
-
Microporation is an efficient method for siRNA-induced knockdown of PEX5 in HepG2 cells: evaluation of the transfection efficiency, the PEX5 mRNA and protein levels and induction of peroxisomal deficiency
Histochemistry and Cell Biology (2014)