Abstract
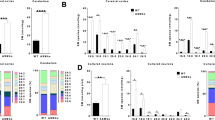
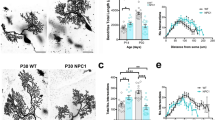
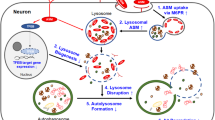
Types A and B Niemann–Pick disease (NPD) result from the deficient activity of acid sphingomyelinase (ASM). An animal model of NPD has been created by gene targeting. In affected animals, the disease followed a severe, neurodegenerative course and death occurred by eight months of age. Analysis of these animals showed their tissues had no detectable ASM activity, the blood cholesterol levels and sphingomyelin in the liver and brain were elevated, and atrophy of the cerebellum and marked deficiency of Purkinje cells was evident. Microscopic analysis revealed NPD ‘cells’ in reticuloendothelial organs and characteristic NPD lesions in the brain. Thus, the ASM deficient mice should be of great value for studying the pathogenesis and treatment of NPD, and for investigations into the role of ASM in signal transduction and apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kanfer, J.N., Young, O.M., Shapiro, D. & Brady, R.O. The metabolism of sphingomyelin. I. Purification and properties of a sphingomyelin-cleaving enzyme from rat liver tissue. J. biol. Chem. 241, 1081–1084 (1966).
Brady, R.O., Kanfer, J.N., Mock, M.B. & Fredrickson, D.S. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick disease. Proc. natn. Acad. Sci. U.S.A. 55, 366–369 (1966).
Schneider, P.B. & Kennedy, E.P. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J. Lipid Res. 8, 202–209 (1967).
Schuchman, E.H. & Desnick, R.J. Niemann-Pick disease Types A and B:Acid sphingomyelinase deficiencies. in The Metabolic and Molecular Bases of Inherited Disease (eds Scriver, C. R., Beaudet, A.L., Sly, W.S.& Valle, D.) 2601–2624 (McGraw-Hill, New York, 1994).
Kolesnick, R. & Golde, D.W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell 77, 325–328 (1994).
Hannun, Y.A. The sphingomyelin cycle and the second messenger function of ceramide. J. biol. Chem. 269, 3125–3128 (1994).
Rao, B.G. & Spence, M.W. Sphingomyelinase activity at pH 7. 4 in human brain and a comparison to activity at pH 5.0. J. Lipid Res. 17, 506–575 (1976).
Yedger, S. & Gatt, S. Effect of Triton X-100 on the hydrolysis of sphingomyelin by sphingomyelinase of rat brain. Biochemistry 15, 2570–2573 (1976).
Gatt, S., Dinur, T. & Kopolvic, J. Niemann-Pick disease: Presence of the magnesium-dependent sphingomyelinase in brain of the infantile form of the disease. J. Neurochem. 31, 547–550 (1978).
Maruyama, E. & Arima, M. Purification and characterization of neutral and acid sphingomyelinases from rat brain. J. Neurochem. 52, 611–618 (1989).
Schuchman, E.H., Suchi, M., Takahashi, T., Sandhoff, K. & Desnick, R.J. Human acid sphingomyelinase. Isolation, nucleotide sequence and expression of the full-length and alternatively spliced RNAs. J. biol. Chem. 66, 8531–8539 (1991).
Schuchman, E.H., Levran, O. & Desnick, R.J. Structural organization and complete nucleotide sequence of the gene encoding human acid sphingomyelinase. Genomics 12, 197–205 (1992).
Newrzella, D. & Stoffel, W. Molecular cloning of the acid sphingomyelinase of the mouse and the organization and complete nucleotide sequence of the gene. Biol. chem. Hoppe-Seyler 373, 1233–1236 (1992).
Pereira, L., Desnick, R.J., Adler, D., Disteche, C.M. & Schuchman, E.H. Regional assignment of the human acid sphingomyelinase gene by PCR analysis of somatic cell hybrids and in situ hybridization to 11 p15.1–p15.4. Genomics 9, 229–234 (1991).
Horinouchi, K., Sakiyama, T., Pereira, L., Lalley, P.A. & Schuchman, E.H. Mouse models of Niemann-Pickdisease: mutation analysis and chromosomal mapping rule out the type A and B forms. Genomics 18, 450–451 (1993).
Suchi, M. et al. Retroviral-mediated transfer of the human acid sphingomyelinase cDNA: Correction of the metabolic defect in cultured Niemann-Pick disease cells. Proc. natn. Acad. Sci. U.S.A. 89, 3227–3231 (1992).
Dinur, T. et al. Toward gene therapy for Niemann-Pick disease (NPD): Separation of retrovirally corrected and noncorrected NPD fibroblasts using a novel fluorescent sphingomyelin. Hum. gene Ther. 3, 633–639 (1992).
Mansour, S.L., Thomas, K.R. & Capecchi, M.R. Disruption of the proto-oncogene int-2 mouse embryo-derived stem cells: A general strategy for targeting mutations to non-selectable genes. Nature 336, 348–352 (1988).
Saiki, R.K. . et al. Primer directed amplification of DNA with a thermostable DNA polymerase. Science 239, 487–491 (1988).
Sanger, F., Nicklen, J. & Coulson, A.R. DNA sequencing with chain terminating inhibitors. Proc. natn. Acad. Sci. U.S.A. 74, 5463–5467 (1977).
Thompson, S., Clarke, A.R., Pow, A.M., Hooper, M.L. & Melton, D.W. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell 56, 313–321 (1989).
Stewart, C., Schuetz, S., Vanek, M. & Wagner, E. Expression of retroviral vectors in transgenic mice obtained by embryo infection. EMBO J. 6, 383–388 (1987).
Kontgen, F. & Stewart, C. A simple screening procedure to detect gene targeting events in embryonic stem cells. Meth. Enzym. 225, 878–890 (1993).
Sambrook, J., Fritsch, E.F. & Maniatis, T., Molecular Cloning: A laboratory manual. (Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, 1989).
Stewart, C.L. Production of chimeras between embryonic stem cells and embryos. Meth. Enzym. 225, 825–855 (1993).
Chatot, C.L., Ziomek, C.A., Bavister, B.D., Lewis, J.L. & Torres, I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J. Reprod. Fert. 86, 679–688 (1989).
Quintern, L.E. & Sandhoff, K. Human acid sphingomyelinase from human urine. Meth. Enzym. 197, 536–540 (1991).
Bishop, D.F. & Desnick, R.J. Affinity purification of α-galactosidase A from human spleen, placenta and plasma with elimination of pyrogen contamination. Properties of the purified splenic enzyme and comparison of other forms. J. biol. Chem. 255, 1307–1316 (1981).
Kieft, K.A., Bocan, T.M.A. & Kraues, B.R. Rapid on-line determination of cholesterol distribution among plasma lipoproteins after high-performance gel-filtration chromatography. J. Lipid Res. 32, 859–866 (1991).
Aalto-Setala, K. et al. Intestinal expression of human apolipoprotein A-IV in transgenic mice fails to influence dietary lipid absorption or feeding behavior. J. clin. Invest. 93, 1776–1786 (1994).
Allain, C.C., Poon, L.S., Chan, C.S.G., Richmond, W. & Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 20, 470–475 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Horinouchi, K., Erlich, S., Perl, D. et al. Acid sphingomyelinase deficient mice: a model of types A and B Niemann–Pick disease. Nat Genet 10, 288–293 (1995). https://doi.org/10.1038/ng0795-288
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0795-288