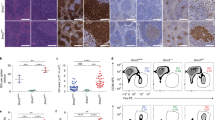
Abstract
Structural alterations of the promoter region of the BCL-6 proto-oncogene represent the most frequent genetic alteration associated with non-Hodgkin lymphoma, a malignancy often deriving from germinal-centre B cells. The BCL-6 gene encodes a zinc-finger transcriptional represser normally expressed in both B cells and CD4+ T cells within germinal centres, but its precise function is unknown. We show that mice deficient in BCL-6 displayed normal B-cell, T-cell and lymphoid-organ development but have a selective defect in T-cell-dependent antibody responses. This defect included a complete lack of affinity maturation and was due to the inability of follicular B cells to proliferate and form germinal centres. In addition, BCL-6-deficient mice developed an inflammatory response in multiple organs characterized by infiltrations of eosinophils and IgE-bearing B lymphocytes typical of a Th2-mediated hyperimmune response. Thus, BCL-6 functions as a transcriptional switch that controls germinal centre formation and may also modulate specific T-cell-mediated responses. Altered expression of BCL-6 in lymphoma represents a deregulation of the pathway normally leading to B cell proliferation and germinal centre formation.
Similar content being viewed by others
Article PDF
References
Ye, B.H., Rao, P.H., Chaganti, R.S.K. & Dalla-Favera, R. Cloning of bcl-6, the locus involved in chromosomal translations affecting band 3q27 in B-cell lymphoma. Cancer Res. 53, 2732–2735 (1993).
Ye, B.H. et al. Alterations of a zinc-finger encoding gene, BCL-6, in diffuse large-cell lymphoma. Science 262, 747–750 (1993).
Baron, B.W. et al. Identification of the gene associated with the recurring chromosomal translocations t(3;14)(q27;q32) and t(3;22)(q27;q11) in B-cell lymphomas. Proc. Natl. Acad. Sci. USA 90, 5262–5266 (1993).
Deweindt, C. et al. Cloning of a breakpoint cluster region at band 3q27 involved in human non-Hodgkin's lymphoma. Genes Chromosomes Cancer 8, 149–154 (1993).
Kerckaert, J.-P. et al. LAZ3, a novel zinc-finger encoding gene, is disrupted by recurring chromosome 3q27 translocations in human lymphoma. Nature Genet. 5, 66–70 (1993).
Miki, T., Kawamata, N., Hirosawa, S. & Aoki, N. Gene involved in the 3q27 translation associated with B-cell lymphoma, BC15, encodes a Krüppel-like zinc-finger protein. Blood 83, 26–32 (1994).
Lo Coco, F. et al. Rearrangements of the BCL-6 gene in diffuse large-cell non-Hodgkin lymphoma. Blood 83, 1757–1759 (1994).
Bastard, C. et al. LAZ3 rearrangements in non-Hodgkin's lymphoma: Correlation with histology, immunophenotype, karyotype, and clinical outcome in 217 patients. Blood 83, 2423–2427 (1994).
Otsuki, T. et al. Analysis of LAZ3 (BCL-6) status in B-cell non-Hodgkin's lymphomas: Results of rearrangement and gene expression studies and a mutational analysis of coding region sequences. Blood 85, 2877–2884 (1995).
Ye, B.H. et al. Chromosomal translocations cause deregulated BCL6 expression by promoter substitution in B cell lymphoma. EMBO J. 14, 6209–6217 (1995).
Migliazza, A. et al. Frequent somatic hypermutation of the 5′ non-coding region of the BCL-6 gene in B-cell lymphoma. Proc. Natl. Acad. Sci. USA. 92, 12520–12524 (1995).
Gaidano, G. et al. Frequent mutation of the 5′ non-coding region of the BCL-6 gene in AIDS-related non-Hodgkin's lymphomas. Blood (in the press).
Dalla-Favera, R. et al. BCL-6 in diffuse large-cell lymphomas. In: Important Advances in Oncology 1996. (eds DeVrta, V. T., Hellman, S. & Rosenberg, S.A.) 139–148 (Lippincott, Philadelphia. 1996).
Chang, C.-C., Ye, B.H., Chaganti, R.S.K. & Dalla-Favera, R. BCL-6, a POZ/Zinc-finger protein, is a sequence specific transcription represser. Proc. Natl. Acad. Sci. USA 93, 6947–6952 (1996).
Seyfert, V.L., Allman, D., He, Y. & Staudt, L.M. Transcriptional repression by the proto-oocogene BCL-6. Oncogens 12, 2331–2342 (1996).
Albagli, O., Dhordain, P., Deweindt, C., Lecocq, G. & Leprince, D. The BTB/POZ domain: a new protein-protein interaction motif common to DMA- and actin-binding proteins. Cell Growth & Differ. 6, 1193–1197 (1995).
Harrison, S.D. & Travers, A.A. Tramtrack gene encodes a Droiophila finger protein that interacts with the ftz transcriptional regulatory region and shows a novel embryonic expression pattern. EMBO J. 9, 207–216 (1990).
DiBello, P.R., Withers, D.A., Bayer, C.A., Fristrom, J.W. & Guild, G.M. Broad-Complex encodes a family of related proteins containing zinc fingers. Genetics 129, 385–397 (1991).
Chardin, P., Courtois, G., Mattel, M.G. & Gisselbrecht, S. Pgene, located on human chromosome 14, encodes a protein with two distant zinc fingers. Nucleic Acid Res. 19, 1431–1436 (1991).
Bardwell, V.J. & Treisman, R. The POZ domain: a conserved protein-protein interation motif. Genes & Dev. 8, 1664–1677 (1994).
Chen, Z. et al. Fusion between a novel Kruppel-like zinc finger gene and the retinoic acid receptor-a locus due to a variant t(11;17) translation associated with acute promyelocytic leukemia. EMBO J. 12, 1161–1167 (1993).
Koonin, E.V., Senkevich, T.G. & Chernos, V.I. A family of DNA virus genes that consists of fused portions of unrelated cellular genes. Trends Biochem. Sci. 17, 213–214 (1992).
Xue, F. & Cooley, L. Kelch encodes a component of intercellular bridges in Drosophila egg chamber. Cell 72, 681–693 (1993).
Deweindt, C. et al. The LAZ3/BCL6 oncogene encodes a sequence-specific transcriptional inhibitor a novel function for the BTB/POZ domain as an autonomous repressing domain. Cell Growth Differ. 6, 1495–1503 (1995).
Cattoretti, G. et al. The BCL-6 protein is expressed in germinal centre B-cells. Blood 86, 45–53 (1995).
Allman, D. et al. BCL-6 expression during B-cell activation. Blood 87, 5257–5268 (1996).
Onizuka, T. et al. BCL-6 gene product, a 92-98-kD nuclear phosphoprotein, is highly expressed in germinal centre B cells and their neoplastic counterparts. Blood 86, 28–37 (1995).
Flenghi, L. et al. Monoclonal antibodies PG-B6a and PG-B6p recognize, respectively, a highly conserved and a formol-resistant eprtope on the human BCL-6 protein arnino-terminal region. Am. J. Pathol. 148, 1543–1555 (1996).
Dhordain, P. et al. The BTB/POZ domain targets the LAZ3/BCL6 oncoprotein to nuclear dots and mediates homomerisation in vivo. Oncogene 11, 2689–2697 (1995).
Murray, A.B. & Luz, A. Acidophilic macrophage pneumonia in laboratory mice. Vet. Pathol. 27, 274–281 (1990).
Abbas, A.K., Murphy, K.M. & Sher, A. Functional diversity of helper T lymphoctes. Nature 383, 787–793 (1996).
Hardy, R.R. & Hayakawa, K. B-lineage differentiation stages resolved by multiparameter flow cytometry. Ann. N. Y. Acad. Sci. USA 764, 19–24 (1995).
Wells, S.M., Kantor, A.B. & Stall, A.M. CD43 (S7) expression identifies peripheral B cell subsets. J. Immunol. 153, 5503–5515 (1994).
Liu, Y.J., Johnson, G.D., Gordon, J. & MacLennan, I. Germinal centres in T-cell-dependent antibody responses. Immunol. Today 13, 17–21 (1992).
Küppers, R., Zhao, M., Hansmann, M.-L. & Rajewsky, K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 12, 4955–4967 (1993).
Rose, M.L., Birbeck, M.S., Wallis, V.J., Forrester, J.A. & Davies, A.J. Peanut letin binding properties of germinal centres of mouse lymphoid tussue. Nature 284, 364–366 (1980).
Orazi, A., Du, X., Yang, Z., Kashai, M. & Williams, D.A. lnterleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab. Invest. 75, 33–42 (1996).
Mombaerts, P. et al. RAG-1-dificient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992).
Snapper, C.M., Finkelman, F.D. & Paul, W.E. Regulation of lgG1 and IgE production by interleukin 4. Immunol. Rev. 102, 51–75 (1988).
Del Prete, G., et al. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatant. J. Immunol. 140, 4193–4198 (1988).
Koike, M. & Takatsu, Y. IL-5 and its receptor which role do they play in the immune response? Int Arch .Allergy Immunol. 104, 1–9 (1994).
Mosmann, T.R. & Coffman, R.L. Th1 and Th2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989).
Hong, R. Diseases due to immunologic deficiency. In: Nelson Textbook of Pediatrics. Edn.13. (eds Behrman, R.E., Vaughan III, V.C. & Nelson. W.E.) 461–466 (W.B. Saunders Company, Philadelphia, 1987).
Ishizaka, A. et al. Regulation of IgE and lgG4 synthesis in patients with hyper IgE syndrome. Immunol. 70, 414–416 (1990).
Crabbe, P.A., Nash, D.R., Bazin, H., Eyssen, H. & Heremans, J.F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. lab. Invest. 22, 448–452 (1970).
Kawabe, T. et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal centre formation. Immunity 1, 167–178 (1994).
Matsumoto, M. et al. Role of rymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 271, 1289–1291 (1996).
Renshaw, B.R. et al. Humoral immune responses in CD40 ligand-dificient mice. J. Exp. Med. 180, 1889–1900 (1994).
Rickert, R.C., Rajewsky, K. & Roes, J. Impairment of T-cell-dependent B-Cell response and B-1 cell development in CD19-deficient mice. Nature 376>352–376 (1995).
Hibbs, M.L. et al. Multiple defects in the immune system of iyn-deficient mice, culminating in autoimmune disease. Cell, 83, 301–311 (1995).
Schwarz, E.M., Krimpenfort, P., Bems, A. & Verma, I.M. Immunological defects in mice with a targeted disruption in Bcl-3. Genes & Dev. 11, 187–197 (1997).
Schubart, D.B., Relink, A., Kosco-Vilbois, M.H., Botteri, F. & Matthias, P. B-cell-specific coactivator OBF-1/OCA-B/Bob1 required for immune response and germinal centre formation. Nature 383, 538–542 (1996).
Kim, U. et al. The B-cell-specific transcription coactivator OCA-B/OBF-1/Bob-1 is essential for normal production of immunoglobulin isotypes. Nature 383, 542–547 (1996).
Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350, 423–426 (1991).
Mombaerts, P. Lymphocyte development and function in T-cell receptor and RAG-1 mutant mice. Int. Rev. Immunol. 13, 43–63 (1995).
Stein, H. & F Diffuse large cell lymphomas of B and T cell type.In: Neoplastic hematopathology. (ed. Knowles, D.M.) 675–714 (Williams & Wilkins. Baltimore. 1992).
Tybulewicz, V.L., Crawford, C.E., Jackson, P.K., Bronson, R.T. & Mulligan, R.C. Neonatal lethality and lymphopenia in mice with a homozyous disruption of the c-abl proto-oncogene. Cell 65, 1153–1163 (1991).
Swiatek. PJ. & Gridley, T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 7, 2071 2084 (1993).
Mortensen, R.M., Conner, D.A., Chao, S., Geisterfer-Lowrance, A.A.T. & Seidman, J.G. Production of homozygous mutant ES cells with a single targeting construct. Mol. Cell. Biol. 12, 2391–2395 (1992).
Stall, A.M. & Wells, S.M. FACS analysis of murine B cell populations.In: The Handbook of Experimental Immunology, Edn. 5. (eds Weir, D.M., Herzenberg, L.A., Blackwell, C.C. & Herzenberg. LA.) Vol. 2, Chapter 63 (Blackwell, Edinburgh, 1997).
Lam, K.-P. & Stall, A.M. Major histocompatability complex class II expression distinguishes two distinct B cell developmental pathways during ontogeny. J. Exp Med. 180, 507–517 (1994).
Roes, J. & Rajewsky, K. Bcl-2 increases memory B cell recruitment but does not perturb selection in germinal centers. J. Exp. Med. 177, 45–55 (1993).
Luna, L.G. Manual of Histologic Staining Methods (MacGraw-Hill, New York, 1960).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, B., Cattoretti, G., Shen, Q. et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet 16, 161–170 (1997). https://doi.org/10.1038/ng0697-161
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0697-161