Abstract
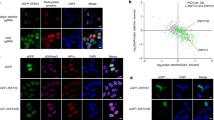
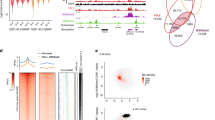
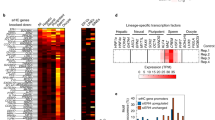
HP1 enrichment at pericentric heterochromatin is considered important for centromere function. Although HP1 binding to H3K9me3 can explain its accumulation at pericentric heterochromatin, how it is initially targeted there remains unclear. Here, in mouse cells, we reveal the presence of long nuclear noncoding transcripts corresponding to major satellite repeats at the periphery of pericentric heterochromatin. Furthermore, we find that major transcripts in the forward orientation specifically associate with SUMO-modified HP1 proteins. We identified this modification as SUMO-1 and mapped it in the hinge domain of HP1α. Notably, the hinge domain and its SUMOylation proved critical to promote the initial targeting of HP1α to pericentric domains using de novo localization assays, whereas they are dispensable for maintenance of HP1 domains. We propose that SUMO-HP1, through a specific association with major forward transcript, is guided at the pericentric heterochromatin domain to seed further HP1 localization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Grewal, S.I. & Jia, S. Heterochromatin revisited. Nat. Rev. Genet. 8, 35–46 (2007).
Guenatri, M., Bailly, D., Maison, C. & Almouzni, G. Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J. Cell Biol. 166, 493–505 (2004).
Probst, A.V., Dunleavy, E. & Almouzni, G. Epigenetic inheritance during the cell cycle. Nat. Rev. Mol. Cell Biol. 10, 192–206 (2009).
Bannister, A.J. et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410, 120–124 (2001).
Lachner, M., O'Carroll, D., Rea, S., Mechtler, K. & Jenuwein, T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410, 116–120 (2001).
Strahl, B.D. & Allis, C.D. The language of covalent histone modifications. Nature 403, 41–45 (2000).
Jenuwein, T. & Allis, C.D. Translating the histone code. Science 293, 1074–1080 (2001).
Peng, H., Ivanov, A.V., Oh, H.J., Lau, Y.F. & Rauscher, F.J. 3rd. Epigenetic gene silencing by the SRY protein is mediated by a KRAB-O protein which recruits the KAP1 co-repressor machinery. J. Biol. Chem. 284, 35670–35680 (2009).
Quivy, J.P. et al. A CAF-1 dependent pool of HP1 during heterochromatin duplication. EMBO J. 23, 3516–3526 (2004).
Lomberk, G., Bensi, D., Fernandez-Zapico, M.E. & Urrutia, R. Evidence for the existence of an HP1-mediated subcode within the histone code. Nat. Cell Biol. 8, 407–415 (2006).
Maison, C. et al. Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nat. Genet. 30, 329–334 (2002).
Muchardt, C. et al. Coordinated methyl and RNA binding is required for heterochromatin localization of mammalian HP1alpha. EMBO Rep. 3, 975–981 (2002).
Rudert, F., Bronner, S., Garnier, J.M. & Dolle, P. Transcripts from opposite strands of gamma satellite DNA are differentially expressed during mouse development. Mamm. Genome 6, 76–83 (1995).
Lu, J. & Gilbert, D.M. Proliferation-dependent and cell cycle regulated transcription of mouse pericentric heterochromatin. J. Cell Biol. 179, 411–421 (2007).
Kalitsis, P. & Choo, K.H.A. Centromere DNA of higher eukaryotes. in The Centromere (ed. Choo, K.H.A.) 97–140 (Oxford University Press, New York, 1997).
Heard, E. & Bickmore, W. The ins and outs of gene regulation and chromosome territory organisation. Curr. Opin. Cell Biol. 19, 311–316 (2007).
Deng, Z., Norseen, J., Wiedmer, A., Riethman, H. & Lieberman, P.M. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol. Cell 35, 403–413 (2009).
Wang, Q., Zhang, Z., Blackwell, K. & Carmichael, G.G. Vigilins bind to promiscuously A-to-I-edited RNAs and are involved in the formation of heterochromatin. Curr. Biol. 15, 384–391 (2005).
Kuroda, M.I., Kernan, M.J., Kreber, R., Ganetzky, B. & Baker, B.S. The maleless protein associates with the X chromosome to regulate dosage compensation in Drosophila. Cell 66, 935–947 (1991).
Irvine, K., Stirling, R., Hume, D. & Kennedy, D. Rasputin, more promiscuous than ever: a review of G3BP. Int. J. Dev. Biol. 48, 1065–1077 (2004).
Shin, J.A. et al. SUMO modification is involved in the maintenance of heterochromatin stability in fission yeast. Mol. Cell 19, 817–828 (2005).
Aagaard, L. et al. Functional mammalian homologues of the Drosophila PEV- modifier Su(Var)3–9 encode centromere-associated proteins which complex with the heterochromatin component M31. EMBO J. 18, 1923–1938 (1999).
Hay, R.T. SUMO: a history of modification. Mol. Cell 18, 1–12 (2005).
Meulmeester, E. & Melchior, F. Cell biology: SUMO. Nature 452, 709–711 (2008).
Park-Sarge, O.K. & Sarge, K.D. Detection of SUMOylated proteins. Methods Mol. Biol. 464, 255–265 (2009).
Jakobs, A. et al. Ubc9 fusion-directed SUMOylation (UFDS): a method to analyze function of protein SUMOylation. Nat. Methods 4, 245–250 (2007).
Peters, A.H. et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337 (2001).
Loyola, A., Bonaldi, T., Roche, D., Imhof, A. & Almouzni, G. PTMs on H3 variants before chromatin assembly potentiate their final epigenetic state. Mol. Cell 24, 309–316 (2006).
Smothers, J.F. & Henikoff, S. The hinge and chromoshadow domain impart distinct targeting of HP1-like proteins. Mol. Cell. Biol. 21, 2555–2569 (2001).
Kerscher, O. SUMO junction-what's your function? New insights through SUMO-interacting motifs. EMBO Rep. 8, 550–555 (2007).
Ouyang, J., Shi, Y., Valin, A., Xuan, Y. & Gill, G. Direct binding of CoREST1 to SUMO-2/3 contributes to gene-specific repression by the LSD1/CoREST1/HDAC complex. Mol. Cell 34, 145–154 (2009).
Allshire, R.C. & Karpen, G.H. Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat. Rev. Genet. 9, 923–937 (2008).
Moazed, D. Small RNAs in transcriptional gene silencing and genome defence. Nature 457, 413–420 (2009).
Blankenship, J.T. & Wieschaus, E. Two new roles for the Drosophila AP patterning system in early morphogenesis. Development 128, 5129–5138 (2001).
Kanellopoulou, C. et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 19, 489–501 (2005).
Murchison, E.P., Partridge, J.F., Tam, O.H., Cheloufi, S. & Hannon, G.J. Characterization of Dicer-deficient murine embryonic stem cells. Proc. Natl. Acad. Sci. USA 102, 12135–12140 (2005).
Tsai, M.C. et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693 (2010).
Savarese, F., Flahndorfer, K., Jaenisch, R., Busslinger, M. & Wutz, A. Hematopoietic precursor cells transiently reestablish permissiveness for X inactivation. Mol. Cell. Biol. 26, 7167–7177 (2006).
Agrelo, R. et al. SATB1 defines the developmental context for gene silencing by Xist in lymphoma and embryonic cells. Dev. Cell 16, 507–516 (2009).
Santos, F., Peters, A.H., Otte, A.P., Reik, W. & Dean, W. Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Dev. Biol. 280, 225–236 (2005).
Puschendorf, M. et al. PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat. Genet. 40, 411–420 (2008).
Probst, A.V. et al. A strand-specific burst in transcription of pericentric satellites is required for chromocenter formation and early mouse development. Dev. Cell 19, 625–638 (2010).
Haaf, T. & Ward, D.C. Higher order nuclear structure in mammalian sperm revealed by in situ hybridization and extended chromatin fibers. Exp. Cell Res. 219, 604–611 (1995).
Mayer, R. et al. Common themes and cell type specific variations of higher order chromatin arrangements in the mouse. BMC Cell Biol. 6, 44 (2005).
Terranova, R., Sauer, S., Merkenschlager, M. & Fisher, A.G. The reorganisation of constitutive heterochromatin in differentiating muscle requires HDAC activity. Exp. Cell Res. 310, 344–356 (2005).
Hajkova, P. et al. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 452, 877–881 (2008).
Solovei, I. et al. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137, 356–368 (2009).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Nielsen, A.L. et al. Heterochromatin formation in mammalian cells: interaction between histones and HP1 proteins. Mol. Cell 7, 729–739 (2001).
Dodson, R.E. & Shapiro, D.J. Vigilin, a ubiquitous protein with 14 K homology domains, is the estrogen-inducible vitellogenin mRNA 3′-untranslated region-binding protein. J. Biol. Chem. 272, 12249–12252 (1997).
Lehnertz, B. et al. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr. Biol. 13, 1192–1200 (2003).
Martini, E., Roche, D.M., Marheineke, K., Verreault, A. & Almouzni, G. Recruitment of phosphorylated chromatin assembly factor 1 to chromatin after UV irradiation of human cells. J. Cell Biol. 143, 563–575 (1998).
Fevrier, B. et al. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. USA 101, 9683–9688 (2004).
Poullet, P., Carpentier, S. & Barillot, E. myProMS, a web server for management and validation of mass spectrometry-based proteomic data. Proteomics 7, 2553–2556 (2007).
Matic, I. et al. In vivo identification of human small ubiquitin-like modifier polymerization sites by high accuracy mass spectrometry and an in vitro to in vivo strategy. Mol. Cell. Proteomics 7, 132–144 (2008).
Acknowledgements
We thank A. Bird, D. Shapiro, R. Hay, T. Jenuwein, R. Losson and J. Seeler for constructs and reagents. We thank W. Faigle for mass spectrometry support, G. Cappello for helpful discussions, A. Cook for critical reading and P. Le Baccon at the Curie Imaging Platform. G.A.'s laboratory is funded by la Ligue Nationale contre le Cancer (Equipe labellisée la Ligue), Curie Programme Incitatif et Collaboratif (PIC) Programs, the European Network of Excellence Epigenome (LSHG-CT-2004-503433), ACI-2007-Cancéropôle IdF 'Breast cancer and Epigenetics', Agence Nationale de la Recherche (ANR) 'EcenS' ANR-09-BLAN-0257-01, INCa 'GepiG', European Research Council (ERC) Advanced Grant 2009-AdG-20090506, and D.L.'s laboratory was funded by le Cancéropôle Ile-de-France and l'INCA.
Author information
Authors and Affiliations
Contributions
C.M., J.-P.Q. and G.A. conceived and designed the experiments. C.M., D.B. and J.-P.Q. performed most of the experiments. D.R. and I.V. performed immuno-DNA FISH and immuno-RNA FISH. A.V.P. performed RNA FISH. F.D., B.L. and D.L. performed and analyzed mass spectrometry data using samples prepared by R.M.d.O. and they wrote together the corresponding parts. C.M. generated all figures. C.M., J.-P.Q. and G.A. analyzed the data. C.M. and G.A. wrote the paper. All authors contributed to final editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1 and 2. (PDF 7708 kb)
Rights and permissions
About this article
Cite this article
Maison, C., Bailly, D., Roche, D. et al. SUMOylation promotes de novo targeting of HP1α to pericentric heterochromatin. Nat Genet 43, 220–227 (2011). https://doi.org/10.1038/ng.765
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.765
This article is cited by
-
Systematic characterization of chromodomain proteins reveals an H3K9me1/2 reader regulating aging in C. elegans
Nature Communications (2023)
-
Satellite repeat transcripts modulate heterochromatin condensates and safeguard chromosome stability in mouse embryonic stem cells
Nature Communications (2022)
-
Dicer promotes genome stability via the bromodomain transcriptional co-activator BRD4
Nature Communications (2022)
-
Unravelling HP1 functions: post-transcriptional regulation of stem cell fate
Chromosoma (2021)
-
Homotypic clustering of L1 and B1/Alu repeats compartmentalizes the 3D genome
Cell Research (2021)