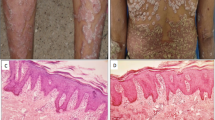
Abstract
Psoriasis is a common inflammatory skin disease with a prevalence of 2–3% in individuals of European ancestry1. In a genome-wide search for copy number variants (CNV) using a sample pooling approach, we have identified a deletion comprising LCE3B and LCE3C, members of the late cornified envelope (LCE) gene cluster2. The absence of LCE3B and LCE3C (LCE3C_LCE3B-del) is significantly associated (P = 1.38E–08) with risk of psoriasis in 2,831 samples from Spain, The Netherlands, Italy and the United States, and in a family-based study (P = 5.4E–04). LCE3C_LCE3B-del is tagged by rs4112788 (r 2 = 0.93), which is also strongly associated with psoriasis (P < 6.6E–09). LCE3C_LCE3B-del shows epistatic effects with the HLA-Cw6 allele on the development of psoriasis in Dutch samples and multiplicative effects in the other samples. LCE expression can be induced in normal epidermis by skin barrier disruption and is strongly expressed in psoriatic lesions, suggesting that compromised skin barrier function has a role in psoriasis susceptibility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lebwohl, M. Psoriasis. Lancet 361, 1197–1204 (2003).
Jackson, B. et al. Late cornified envelope family in differentiating epithelia–response to calcium and ultraviolet irradiation. J. Invest. Dermatol. 124, 1062–1070 (2005).
Krueger, J.G. & Bowcock, A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann. Rheum. Dis. 64 Suppl 2, ii30–ii36 (2005).
Lowes, M.A., Bowcock, A.M. & Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 445, 866–873 (2007).
Bowcock, A.M. & Cookson, W.O. The genetics of psoriasis, psoriatic arthritis and atopic dermatitis. Hum. Mol. Genet. 13 (Spec. No. 1) R43–R55 (2004).
Russell, T.J., Schultes, L.M. & Kuban, D.J. Histocompatibility (HL-A) antigens associated with psoriasis. N. Engl. J. Med. 287, 738–740 (1972).
Elder, J.T. PSORS1: linking genetics and immunology. J. Invest. Dermatol. 126, 1205–1206 (2006).
Liu, Y. et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 4, e1000041 (2008).
Nair, R.P. et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet. 78, 827–851 (2006).
Cargill, M. et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 80, 273–290 (2007).
Hollox, E.J. et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat. Genet. 40, 23–25 (2008).
Redon, R. et al. Global variation in copy number in the human genome. Nature 444, 444–454 (2006).
Estivill, X. & Armengol, L. Copy number variants and common disorders: filling the gaps and exploring complexity in genome-wide association studies. PLoS Genet. 3, 1787–1799 (2007).
Capon, F. et al. Searching for psoriasis susceptibility genes in Italy: genome scan and evidence for a new locus on chromosome 1. J. Invest. Dermatol. 112, 32–35 (1999).
Korbel, J.O. et al. Paired-end mapping reveals extensive structural variation in the human genome. Science 318, 420–426 (2007).
Kidd, J.M. et al. Mapping and sequencing of structural variation from eight human genomes. Nature 453, 56–64 (2008).
Zhao, X.P. & Elder, J.T. Positional cloning of skin-specific genes from the human epidermal differentiation complex. Genomics 45, 250–258 (1997).
Chen, H. et al. Association of skin barrier genes within the PSORS4 locus is enriched in Singaporean Chinese with early-onset psoriasis. J. Invest. Dermatol. advance online publication, doi:10.1038/jid.2008.273 (11 September 2008).
Asumalahti, K. et al. Coding haplotype analysis supports HCR as the putative susceptibility gene for psoriasis at the MHC PSORS1 locus. Hum. Mol. Genet. 11, 589–597 (2002).
Nair, R.P. et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J. Invest. Dermatol. 128, 1653–1661 (2008).
Capon, F., Semprini, S., Dallapiccola, B. & Novelli, G. Evidence for interaction between psoriasis-susceptibility loci on chromosomes 6p21 and 1q21. Am. J. Hum. Genet. 65, 1798–1800 (1999).
McCarroll, S.A. et al. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn's disease. Nat. Genet. 40, 1107–1112 (2008).
Aberg, K.M. et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J. Invest. Dermatol. 128, 917–925 (2008).
Gonzalez, J.R. et al. Maximizing association statistics over genetic models. Genet. Epidemiol. 32, 246–254 (2008).
Cordell, H.J. et al. Case/pseudocontrol analysis in genetic association studies: A unified framework for detection of genotype and haplotype associations, gene-gene and gene-environment interactions, and parent-of-origin effects. Genet. Epidemiol. 26, 167–185 (2004).
Alkemade, J.A. et al. SKALP/elafin is an inducible proteinase inhibitor in human epidermal keratinocytes. J. Cell Sci. 107, 2335–2342 (1994).
Acknowledgements
We gratefully acknowledge the participants in the genetic studies whose contributions made this work possible and personnel at the CeGen genotyping core facility (Barcelona node). We thank T. Henseler, S. Jenisch, M. Weichenthal and E. Christophers from the University of Kiel for making samples from Kiel, Germany available for analysis. We thank A. Poon for technical assistance, the University of California San Francisco Psoriasis Center Staff for help with subject recruitment and J. Gartlon for proofreading the manuscript. This work was supported in part by funds from the “Generalitat de Catalunya,” the Spanish Ministry of Science and Innovation and the European Commission ENGAGE Project (X.E., R.d.C., L.A., E.R.-M., G.E. and E.B.), ADIPSO (G.N.) and National Institutes for Arthritis, Musculoskeletal and Skin Diseases (A.B., J.T.E., R.N. and P.E.S.). E.R.-M. is supported by CIBERESP (Carlos III Health Institute). J.T.E. is supported by the Ann Arbor Veterans Affairs Hospital. W.L. is supported by a grant from the Dermatology Foundation.
Author information
Authors and Affiliations
Contributions
R.d.C., E.R.-M., P.L.J.M.Z. and J.R. contributed equally to this work. R.d.C., G.M.-E., R.M.P. and C.L. recruited the subjects from Spain. P.L.J.M.Z., M.K., M.d.H. and J.S. recruited the subjects from The Netherlands. E.G. and G.N. recruited the subjects from Italy. P.-Y.K., A.B., R.N., W.L. and J.T.E. recruited the subjects from the USA. R.d.C., E.R.-M., P.L.J.M.Z., J.R., W.L., E.N.D., E.G., I.J., C.H., E.B., P.E.S. and R.N. performed the genotyping and experimental work. R.d.C., L.A., G.E., G.A., P.E.S., J.S. and X.E. analyzed the data. J.S. was responsible for the design and execution of the expression studies in keratinocytes. X.E., G.N., J.T.E., E.E.E., J.A.L.A., P.K., A.B. and J.S. supervised the work. X.E. designed the study and coordinated the work. All authors contributed to the final version of the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Tables 1–11, Supplementary Methods and Supplementary Note (PDF 906 kb)
Rights and permissions
About this article
Cite this article
de Cid, R., Riveira-Munoz, E., Zeeuwen, P. et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet 41, 211–215 (2009). https://doi.org/10.1038/ng.313
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.313