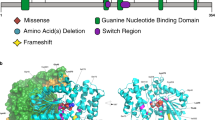
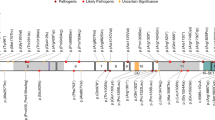
Abstract
Copy number variants (CNVs) are associated with many neurocognitive disorders; however, these events are typically large, and the underlying causative genes are unclear. We created an expanded CNV morbidity map from 29,085 children with developmental delay in comparison to 19,584 healthy controls, identifying 70 significant CNVs. We resequenced 26 candidate genes in 4,716 additional cases with developmental delay or autism and 2,193 controls. An integrated analysis of CNV and single-nucleotide variant (SNV) data pinpointed 10 genes enriched for putative loss of function. Follow-up of a subset of affected individuals identified new clinical subtypes of pediatric disease and the genes responsible for disease-associated CNVs. These genetic changes include haploinsufficiency of SETBP1 associated with intellectual disability and loss of expressive language and truncations of ZMYND11 in individuals with autism, aggression and complex neuropsychiatric features. This combined CNV and SNV approach facilitates the rapid discovery of new syndromes and genes involved in neuropsychiatric disease despite extensive genetic heterogeneity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
Accessions
NCBI Reference Sequence
References
Cooper, G.M. et al. A copy number variation morbidity map of developmental delay. Nat. Genet. 43, 838–846 (2011).
Kaminsky, E.B. et al. An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet. Med. 13, 777–784 (2011).
Moreno-De-Luca, D. et al. Using large clinical data sets to infer pathogenicity for rare copy number variants in autism cohorts. Mol. Psychiatry 18, 1090–1095 (2013).
Vulto-van Silfhout, A.T. et al. Clinical significance of de novo and inherited copy-number variation. Hum. Mutat. 34, 1679–1687 (2013).
Allen, A.S. et al. De novo mutations in epileptic encephalopathies. Nature 501, 217–221 (2013).
de Ligt, J. et al. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 367, 1921–1929 (2012).
Gulsuner, S. et al. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 154, 518–529 (2013).
Iossifov, I. et al. De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285–299 (2012).
Jiang, Y.H. et al. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. Am. J. Hum. Genet. 93, 249–263 (2013).
Neale, B.M. et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485, 242–245 (2012).
O'Roak, B.J. et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250 (2012).
Rauch, A. et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet 380, 1674–1682 (2012).
Sanders, S.J. et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241 (2012).
Zaidi, S. et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature 498, 220–223 (2013).
Jacquemont, S. et al. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am. J. Hum. Genet. 94, 415–425 (2014).
Rudd, M.K. et al. Segmental duplications mediate novel, clinically relevant chromosome rearrangements. Hum. Mol. Genet. 18, 2957–2962 (2009).
Burkardt, D.D. et al. Distinctive phenotype in 9 patients with deletion of chromosome 1q24-q25. Am. J. Med. Genet. A. 155A, 1336–1351 (2011).
Dabell, M.P. et al. Investigation of NRXN1 deletions: clinical and molecular characterization. Am. J. Med. Genet. A. 161A, 717–731 (2013).
Gimelli, S. et al. A rare 3q13.31 microdeletion including GAP43 and LSAMP genes. Mol. Cytogenet. 6, 52 (2013).
Madrigal, I., Martinez, M., Rodriguez-Revenga, L., Carrio, A. & Mila, M. 12p13 rearrangements: 6 Mb deletion responsible for ID/MCA and reciprocal duplication without clinical responsibility. Am. J. Med. Genet. A. 158A, 1071–1076 (2012).
Paciorkowski, A.R. et al. MEF2C haploinsufficiency features consistent hyperkinesis, variable epilepsy, and has a role in dorsal and ventral neuronal developmental pathways. Neurogenetics 14, 99–111 (2013).
Rosenfeld, J.A. et al. Small deletions of SATB2 cause some of the clinical features of the 2q33.1 microdeletion syndrome. PLoS ONE 4, e6568 (2009).
Stankiewicz, P. et al. Recurrent deletions and reciprocal duplications of 10q11.21q11.23 including CHAT and SLC18A3 are likely mediated by complex low-copy repeats. Hum. Mutat. 33, 165–179 (2012).
van Bon, B.W. et al. The phenotype of recurrent 10q22q23 deletions and duplications. Eur. J. Hum. Genet. 19, 400–408 (2011).
Döcker, D. et al. Further delineation of the SATB2 phenotype. Eur. J. Hum. Genet. 22, 1034–1039 (2014).
Thorsson, T. et al. Chromosomal imbalances in patients with congenital cardiac defects: a meta-analysis reveals novel potential critical regions involved in heart development. Congenit. Heart Dis. 10.1111/chd.12179 (11 April 2014).
Le Meur, N. et al. MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J. Med. Genet. 47, 22–29 (2010).
Ching, M.S. et al. Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 153B, 937–947 (2010).
Shuvarikov, A. et al. Recurrent HERV-H–mediated 3q13.2-q13.31 deletions cause a syndrome of hypotonia and motor, language, and cognitive delays. Hum. Mutat. 34, 1415–1423 (2013).
Endele, S. et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat. Genet. 42, 1021–1026 (2010).
Goffin, A., Hoefsloot, L.H., Bosgoed, E., Swillen, A. & Fryns, J.P. PTEN mutation in a family with Cowden syndrome and autism. Am. J. Med. Genet. 105, 521–524 (2001).
Koolen, D.A. et al. Mutations in the chromatin modifier gene KANSL1 cause the 17q21.31 microdeletion syndrome. Nat. Genet. 44, 639–641 (2012).
Lossin, C. A catalog of SCN1A variants. Brain Dev. 31, 114–130 (2009).
Santen, G.W. et al. Mutations in SWI/SNF chromatin remodeling complex gene ARID1B cause Coffin-Siris syndrome. Nat. Genet. 44, 379–380 (2012).
Talkowski, M.E. et al. Assessment of 2q23.1 microdeletion syndrome implicates MBD5 as a single causal locus of intellectual disability, epilepsy, and autism spectrum disorder. Am. J. Hum. Genet. 89, 551–563 (2011).
O'Roak, B.J. et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338, 1619–1622 (2012).
Fischbach, G.D. & Lord, C. The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron 68, 192–195 (2010).
Sharp, A.J. et al. Segmental duplications and copy-number variation in the human genome. Am. J. Hum. Genet. 77, 78–88 (2005).
van Bon, B.W. et al. Intragenic deletion in DYRK1A leads to mental retardation and primary microcephaly. Clin. Genet. 79, 296–299 (2011).
Girirajan, S. et al. Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. Am. J. Hum. Genet. 92, 221–237 (2013).
Filges, I. et al. Reduced expression by SETBP1 haploinsufficiency causes developmental and expressive language delay indicating a phenotype distinct from Schinzel-Giedion syndrome. J. Med. Genet. 48, 117–122 (2011).
Marseglia, G. et al. 372 kb microdeletion in 18q12.3 causing SETBP1 haploinsufficiency associated with mild mental retardation and expressive speech impairment. Eur. J. Med. Genet. 55, 216–221 (2012).
DeScipio, C. et al. Subtelomeric deletion of chromosome 10p15.3: clinical findings and molecular cytogenetic characterization. Am. J. Med. Genet. A. 158A, 2152–2161 (2012).
Ansieau, S. & Leutz, A. The conserved Mynd domain of BS69 binds cellular and oncoviral proteins through a common PXLXP motif. J. Biol. Chem. 277, 4906–4910 (2002).
Kateb, F. et al. Structural and functional analysis of the DEAF-1 and BS69 MYND domains. PLoS ONE 8, e54715 (2013).
Masselink, H. & Bernards, R. The adenovirus E1A binding protein BS69 is a corepressor of transcription through recruitment of N-CoR. Oncogene 19, 1538–1546 (2000).
Stefansson, H. et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature 505, 361–366 (2014).
Hoischen, A. et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat. Genet. 42, 483–485 (2010).
Schinzel, A. & Giedion, A. A syndrome of severe midface retraction, multiple skull anomalies, clubfeet, and cardiac and renal malformations in sibs. Am. J. Med. Genet. 1, 361–375 (1978).
Makishima, H. et al. Somatic SETBP1 mutations in myeloid malignancies. Nat. Genet. 45, 942–946 (2013).
Piazza, R. et al. Recurrent SETBP1 mutations in atypical chronic myeloid leukemia. Nat. Genet. 45, 18–24 (2013).
Wen, H. et al. ZMYND11 links histone H3.3K36me3 to transcription elongation and tumour suppression. Nature 508, 263–268 (2014).
Yu, B. et al. BS69 undergoes SUMO modification and plays an inhibitory role in muscle and neuronal differentiation. Exp. Cell Res. 315, 3543–3553 (2009).
Fromer, M. et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179–184 (2014).
Alliman, S. et al. Clinical and molecular characterization of individuals with recurrent genomic disorder at 10q22.3q23.2. Clin. Genet. 78, 162–168 (2010).
Hehir-Kwa, J.Y. et al. De novo copy number variants associated with intellectual disability have a paternal origin and age bias. J. Med. Genet. 48, 776–778 (2011).
Duker, A.L. et al. Paternally inherited microdeletion at 15q11.2 confirms a significant role for the SNORD116 C/D box snoRNA cluster in Prader-Willi syndrome. Eur. J. Hum. Genet. 18, 1196–1201 (2010).
Hubert, M. & Van der Veeken, S. Outlier detection for skewed data. J. Chemometr. 22, 235–246 (2008).
Rosenfeld, J.A., Coe, B.P., Eichler, E.E., Cuckle, H. & Shaffer, L.G. Estimates of penetrance for recurrent pathogenic copy-number variations. Genet. Med. 15, 478–481 (2013).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J.R. Stat. Soc. 57, 289–300 (1995).
Acknowledgements
We thank F. Hormozdiari, M. Dennis and T. Brown for useful discussions and for editing the manuscript. B.P.C. is supported by a fellowship from the Canadian Institutes of Health Research. This study makes use of data generated by the Wellcome Trust Case Control Consortium. A full list of the investigators who contributed to the generation of the data is available from http://www.wtccc.org.uk/. J.A.R. and B.S.T. are employees of Signature Genomics Laboratories, LLC, a subsidiary of PerkinElmer, Inc. This work was supported by US National Institute of Mental Health grant MH101221 and Paul G. Allen Family Foundation Award 11631 to E.E.E. E.E.E. is an Allen Distinguished Investigator and an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
B.P.C. and E.E.E. designed the study. B.P.C. performed the data analysis. B.P.C., K.W. and C.B. performed array CGH, MIP sequencing and Sanger validation. J.A.R. and B.S.T. supervised array CGH experiments and coordinated clinical data collection at Signature Genomics. B.W.M.v.B., A.T.V.-v.S., P.B., K.L.F., S.B., L.E.L.M.V., J.H.S.-H., A.H., D.L., D.A., N.B., P.J.L., I.E.S., A.A., R. Pettinato, R.T., N.d.L., M.R.F.R., H.P., E.T., M.F., M.S., H.C.M., E.H., C.R., J.G. and B.B.A.d.V. provided clinical samples for resequencing, clinical reports and inheritance testing. J.Y.H.-K., R. Pfundt and N.d.L. curated the Nijmegen de novo CNV calls. B.P.C., K.W., C.B., B.J.O., J.S., and E.E.E. designed the MIP gene panel. G.L.C. and H.C.M. identified two SETBP1 variants in an independent screen. N.K. curated published de novo mutations. B.P.C. and E.E.E. wrote the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.A.R. and B.S.T. are employees of Signature Genomics Laboratories, LLC, a subsidiary of PerkinElmer, Inc. E.E.E. is on the scientific advisory board (SAB) of DNAnexus, Inc., and was an SAB member of Pacific Biosciences, Inc. (2009–2013) and SynapDx Corp. (2011–2013).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Tables 1–3, 5, 8, 9 and 11, and Supplementary Note. (PDF 3450 kb)
Supplementary Tables 4, 6, 7 and 10
Supplementary Tables 4, 6, 7 and 10. (XLSX 3564 kb)
Supplementary Data Set 1
CNV window counts and P values. (XLSX 1111 kb)
Rights and permissions
About this article
Cite this article
Coe, B., Witherspoon, K., Rosenfeld, J. et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet 46, 1063–1071 (2014). https://doi.org/10.1038/ng.3092
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3092
This article is cited by
-
Retrospective file review shows limited genetic services fail most patients – an argument for the implementation of exome sequencing as a first-tier test in resource-constrained settings
Orphanet Journal of Rare Diseases (2023)
-
A novel 1p13.2 deletion associates with neurodevelopmental disorders in a three-generation pedigree
BMC Medical Genomics (2023)
-
Identifying the neurodevelopmental and psychiatric signatures of genomic disorders associated with intellectual disability: a machine learning approach
Molecular Autism (2023)
-
Novel SETBP1 mutation in a chinese family with intellectual disability
BMC Medical Genomics (2023)
-
Outcomes of two different unbalanced segregations from a maternal t(4;10)(q33;p15.1) translocation
BMC Medical Genomics (2023)