Abstract
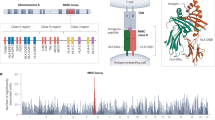
Ankylosing spondylitis is a common, highly heritable inflammatory arthritis affecting primarily the spine and pelvis. In addition to HLA-B*27 alleles, 12 loci have previously been identified that are associated with ankylosing spondylitis in populations of European ancestry, and 2 associated loci have been identified in Asians. In this study, we used the Illumina Immunochip microarray to perform a case-control association study involving 10,619 individuals with ankylosing spondylitis (cases) and 15,145 controls. We identified 13 new risk loci and 12 additional ankylosing spondylitis–associated haplotypes at 11 loci. Two ankylosing spondylitis–associated regions have now been identified encoding four aminopeptidases that are involved in peptide processing before major histocompatibility complex (MHC) class I presentation. Protective variants at two of these loci are associated both with reduced aminopeptidase function and with MHC class I cell surface expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Braun, J., Listing, J. & Sieper, J. Overestimation of the prevalence of ankylosing spondylitis in the Berlin study: comment on the article by Braun et al—Reply. Arthritis Rheum. 52, 4049–4050 (2005).
Ng, S.C. et al. Epidemiology of spondyloarthritis in the People's Republic of China: review of the literature and commentary. Semin. Arthritis Rheum. 37, 39–47 (2007).
Brown, M.A., Laval, S.H., Brophy, S. & Calin, A. Recurrence risk modelling of the genetic susceptibility to ankylosing spondylitis. Ann. Rheum. Dis. 59, 883–886 (2000).
Brown, M.A. et al. Susceptibility to ankylosing spondylitis in twins: the role of genes, HLA, and the environment. Arthritis Rheum. 40, 1823–1828 (1997).
Burton, P.R. et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Evans, D.M. et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 43, 761–767 (2011).
Reveille, J.D. et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat. Genet. 42, 123–127 (2010).
Lin, Z. et al. A genome-wide association study in Han Chinese identifies new susceptibility loci for ankylosing spondylitis. Nat. Genet. 44, 73–77 (2012).
Cortes, A. & Brown, M.A. Promise and pitfalls of the Immunochip. Arthritis Res. Ther. 13, 101 (2011).
Trynka, G. et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nat. Genet. 43, 1193–1201 (2011).
Ferreira, M.A. et al. Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet 378, 1006–1014 (2011).
Melzer, D. et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet. 4, e1000072 (2008).
Mizuki, N. et al. Genome-wide association studies identify IL23R–IL12RB2 and IL10 as Behcet′s disease susceptibility loci. Nat. Genet. 42, 703–706 (2010).
Remmers, E.F. et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behcet's disease. Nat. Genet. 42, 698–702 (2010).
Ait Badi, M.A. et al. Skeletal manifestations in Behcet's disease. A report of 79 cases. Rev. Med. Interne 29, 277–282 (2008).
Harvey, D. et al. A common functional variant of endoplasmic reticulum aminopeptidase 2 (ERAP2) that reduces major histocompatibility complex class I expression is not associated with ankylosing spondylitis. Rheumatology 50, 1720–1721 (2011).
Tsui, F.W. et al. Association of an ERAP1-ERAP2 haplotype with familial ankylosing spondylitis. Ann. Rheum. Dis. 69, 733–736 (2010).
Evnouchidou, I. et al. A common single nucleotide polymorphism in Endoplasmic Reticulum Aminopeptidase 2 induces a specificity switch that leads to altered antigen processing. J. Immunol. 189, 2383–2392 (2012).
Andrés, A.M. et al. Balancing selection maintains a form of ERAP2 that undergoes nonsense-mediated decay and affects antigen presentation. PLoS Genet. 6, e1001157 (2010).
Lévy, F. et al. The final N-terminal trimming of a subaminoterminal proline-containing HLA class I–restricted antigenic peptide in the cytosol is mediated by two peptidases. J. Immunol. 169, 4161–4171 (2002).
Xia, Z. et al. A 17q12 allele is associated with altered NK cell subsets and function. J. Immunol. 188, 3315–3322 (2012).
Trynka, G. et al. Chromatin marks identify critical cell types for fine mapping complex trait variants. Nat. Genet. 45, 124–130 (2013).
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Ferreira, M.A. et al. Quantitative trait loci for CD4:CD8 lymphocyte ratio are associated with risk of type 1 diabetes and HIV-1 immune control. Am. J. Hum. Genet. 86, 88–92 (2010).
Davidson, S.I. et al. Association of ERAP1, but not IL23R, with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum. 60, 3263–3268 (2009).
Kumar, P., Henikoff, S. & Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081 (2009).
Adzhubei, I.A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Sato, K. et al. Strong evidence of a combination polymorphism of the tyrosine kinase 2 gene and the signal transducer and activator of transcription 3 gene as a DNA-based biomarker for susceptibility to Crohn's disease in the Japanese population. J. Clin. Immunol. 29, 815–825 (2009).
Strange, A. et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 42, 985–990 (2010).
Ban, M. et al. Replication analysis identifies TYK2 as a multiple sclerosis susceptibility factor. Eur. J. Hum. Genet. 17, 1309–1313 (2009).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Kenna, T.J. et al. Enrichment of circulating interleukin-17–secreting interleukin-23 receptor–positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 64, 1420–1429 (2012).
Appel, H. et al. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 13, R95 (2011).
Pearce, E.L. et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302, 1041–1043 (2003).
Yagi, R. et al. The transcription factor GATA3 actively represses RUNX3 protein–regulated production of interferon-γ. Immunity 32, 507–517 (2010).
Cruz-Guilloty, F. et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 206, 51–59 (2009).
Intlekofer, A.M. et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321, 408–411 (2008).
Park, J.H. et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat. Immunol. 11, 257–264 (2010).
Rakowski, L.A. et al. Convergence of the ZMIZ1 and NOTCH1 pathways at C-MYC in acute T lymphoblastic leukemias. Cancer Res. 73, 930–941 (2013).
Hartgring, S.A., Willis, C.R., Bijlsma, J.W., Lafeber, F.P. & van Roon, J.A. Interleukin-7 aggravated joint inflammation and tissue destruction in collagen-induced arthritis is associated with T-cell and B-cell activation. Arthritis Res. Ther. 14, R137 (2012).
Muto, A. et al. Bach2 represses plasma cell gene regulatory network in B cells to promote antibody class switch. EMBO J. 29, 4048–4061 (2010).
Song, I.H. et al. Different response to rituximab in tumor necrosis factor blocker–naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trial. Arthritis Rheum. 62, 1290–1297 (2010).
Huang, X., Li, Y., Tanaka, K., Moore, K.G. & Hayashi, J.I. Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase Cγ1, Grb2, and phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 92, 11618–11622 (1995).
Evnouchidou, I. et al. Cutting Edge: Coding single nucleotide polymorphisms of endoplasmic reticulum aminopeptidase 1 can affect antigenic peptide generation in vitro by influencing basic enzymatic properties of the enzyme. J. Immunol. 186, 1909–1913 (2011).
Saveanu, L. et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat. Immunol. 6, 689–697 (2005).
Colbert, R.A. et al. HLA-B27 misfolding activates the IL-23/IL-17 axis via the unfolded protein response in transgenic rats: evidence for a novel mechanism of inflammation. Arthritis Rheum. 1283, S515 (2007).
Karaderi, T. et al. Evidence of genetic association between TNFRSF1A encoding the p55 tumour necrosis factor receptor, and ankylosing spondylitis in UK Caucasians. Clin. Exp. Rheumatol. 30, 110–113 (2012).
Gregory, A.P. et al. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature 488, 508–511 (2012).
Hemminki, K., Li, X., Sundquist, K. & Sundquist, J. Familial association of inflammatory bowel diseases with other autoimmune and related diseases. Am. J. Gastroenterol. 105, 139–147 (2010).
Thjodleifsson, B., Geirsson, A.J., Bjornsson, S. & Bjarnason, I. A common genetic background for inflammatory bowel disease and ankylosing spondylitis: a genealogic study in Iceland. Arthritis Rheum. 56, 2633–2639 (2007).
Danoy, P. et al. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn's disease. PLoS Genet. 6, e1001195 (2010).
Laukens, D. et al. Evidence for significant overlap between common risk variants for Crohn's disease and ankylosing spondylitis. PLoS ONE 5, e13795 (2010).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010).
Sawcer, S. et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476, 214–219 (2011).
Onozawa, Y. et al. Activation of T cell death–associated gene 8 regulates the cytokine production of T cells and macrophages in vitro. Eur. J. Pharmacol. 683, 325–331 (2012).
Ryder, C., McColl, K., Zhong, F. & Distelhorst, C.W. Acidosis promotes Bcl-2 family mediated evasion of apoptosis: involvement of acid-sensing G protein–coupled receptor GPR65 signaling to MEK/ERK. J. Biol. Chem. 287, 27863–27875 (2012).
Khan, M.A., Kushner, I. & Braun, W.E. Association of HLA-A2 with uveitis in HLA-B27 positive patients with ankylosing spondylitis. J. Rheumatol. 8, 295–298 (1981).
Liu, J.B. et al. Association of vitiligo with HLA-A2: a meta-analysis. J. Eur. Acad. Dermatol. Venereol. 21, 205–213 (2007).
Noble, J.A. et al. HLA class I and genetic susceptibility to type 1 diabetes: results from the Type 1 Diabetes Genetics Consortium. Diabetes 59, 2972–2979 (2010).
van der Linden, S., Valkenburg, H.A. & Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 (1984).
Shah, T.S. et al. optiCall: a robust genotype-calling algorithm for rare, low-frequency and common variants. Bioinformatics 28, 1598–1603 (2012).
Fraley, C. & Raftery, A.E. Model-based clustering, discriminant analysis, and density estimation. J. Am. Stat. Assoc. 97, 611–631 (2002).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Lippert, C. et al. FaST linear mixed models for genome-wide association studies. Nat. Methods 8, 833–835 (2011).
Pruim, R.J. et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–2337 (2010).
1000 Genomes Project Consortium.. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Li, Y., Willer, C.J., Ding, J., Scheet, P. & Abecasis, G.R. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol. 34, 816–834 (2010).
Wellcome Trust Case Control Consortium. Genomewide association study of 14,000 cases of seven common diseases and 3000 controls. Nature 447, 661–678 (2007).
Acknowledgements
We thank all participating subjects with ankylosing spondylitis and healthy individuals who provided the DNA and clinical information necessary for this study. The Wellcome Trust Case Control Consortium 2 project is funded by the Wellcome Trust (083948/Z/07/Z). We acknowledge use of the British 1958 Birth Cohort DNA collection, funded by the Medical Research Council (G0000934) and the Wellcome Trust (068545/Z/02), and of the UK National Blood Service controls, funded by the Wellcome Trust. We thank J.C. Barrett for contributing the design of the Immunochip and for helpful analytical discussion, as well as E. Gray, S. Bumpstead, D. Simpkin and the staff of the Wellcome Trust Sanger Institute Sample Management and Genotyping teams for their genotyping and analytical contributions. The Australo-Anglo-American Spondyloarthritis Consortium (TASC) study was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grants P01-052915 and R01-AR046208. Funding was also received from University of Texas at Houston Clinical and Translational Science Award (CTSA) UL1RR024188, Cedars-Sinai General Clinical Research Center (GCRC) grant MO1-RR00425, the Intramural Research Program, NIAMS, US National Institutes of Health and the Rebecca Cooper Foundation (Australia). This study was funded, in part, by Arthritis Research UK (grants 19536 and 18797), by the Wellcome Trust (grant 076113) and by the Oxford Comprehensive Biomedical Research Centre ankylosing spondylitis chronic disease cohort (theme A91202). We thank A. Harrison (University of Otago) for his contribution to the New Zealand ankylosing spondylitis cohort. H.X. was funded by National Natural Science Foundation of China grants 81020108029 and 30872339. Portuguese sample collection was performed by COnhecer a Realidade PORtuguesa sobre a Espondilite Anquilosante (CORPOREA Study Group), coordinated by F.M.P.-S. and supported by Bolsa Investigação da Sociedade Portuguesa de Reumatologia/Schering-Plough 2007. The Spanish ankylosing spondylitis case collection was supported by Spanish grant FICYT PC-10-70-Fondos FEDER European Union. The Spondyloarthritis Research Consortium of Canada (SPARCC) was funded by a National Research Initiative Award from the Arthritis Society (Canada). French sample collection was performed by the Groupe Française d'Etude Génétique des Spondylarthrites, coordinated by R. Said-Nahal and funded by Agence Nationale de Recherche GEnetics, Microbiota, Inflammation and Spondyloarthritis (GEMISA) grant ANR-10-MIDI-0002. We thank the Norwegian Bone Marrow Donor registry for providing data from healthy Norwegian controls. W.P.M. is a Medical Scientist of Alberta Innovates–Health Solutions. The Psoriatic Arthritis Program is supported by the Krembil Foundation and the Arthritis Society. P.C.R. is funded by the National Health and Medical Research Council (Australia) (NHMRC) and Arthritis Australia. J.Y. is supported by NHMRC grants 613672 and 1011506. M. Ward is supported by the Intramural Research Program, NIAMS, US National Institutes of Health. D.E. is supported by the research council of Ghent University and by the Fund for Scientific Research Flanders. M.A.B. is funded by a National Health and Medical Research Council (Australia) Senior Principal Research Fellowship, and support for this study was received from a National Health and Medical Research Council (Australia) program grant (566938) and project grant (569829) and from the Australian Cancer Research Foundation and the Rebecca Cooper Medical Research Foundation. We thank A. Gardiner and the Brisbane Convention and Exhibition Centre for their assistance in preparing the manuscript. We are also very grateful for the invaluable support received from the National Ankylosing Spondylitis Society (UK) and the Spondyloarthritis Association of America in case recruitment. Additional financial and technical support for subject recruitment was provided by the NIHR Oxford Musculoskeletal Biomedical Research Unit and NIHR Thames Valley Comprehensive Local Research and by an unrestricted educational grant from Abbott Laboratories.
Author information
Authors and Affiliations
Consortia
Contributions
J. Hadler, K.C., K.P. and J. Harris performed genotyping. A.C., P.C.R., T.K., P.L., J.Y., M.A.B. and D.M.E. performed statistical analyses. J.P.P., S.L., K.B.J., S.-C.S., M. Weisman, M. Ward, X.Z., H.-J.G., G.C., J.N., B.A.L., Ø.F., J.T., K.L., L.J., Y.L., X.W., L.A.B., D.E., R.B.-V., S.S., L.A., C.F., J.L., N.H., J. Mulero, J.L.F.-S., M.A.G.-G., C.L.-L., P. Deloukas, P. Donnelly, P.B., K.G., H.G., D.D.G., P.R., W.P.M., H.X., J.B.A.C., I.E.v.d.H.-B., C.-T.C., R.V.-O., C.R.-S., I.M.H., F.M.P.-S., R.D.I., V.V., J. Martin, M.B., J.D.R. and T.-H.K. all contributed to subject recruitment and study design. A.C., M.A.B., D.M.E. and B.P.W. wrote the manuscript, and all authors contributed to manuscript drafting and reviewed the final manuscript. T.J.K. performed cell count and IL-6R studies in ankylosing spondylitis cases and controls. M.A.F. performed GWAS of cell counts in controls.
Corresponding author
Ethics declarations
Competing interests
The author declare no competing financial interests.
Additional information
Details appear in the Supplementary Note.
Details appear in the Supplementary Note.
Details appear in the Supplementary Note.
Details appear in the Supplementary Note.
Details appear in the Supplementary Note.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–11, Supplementary Figures 1–12, Supplementary Note (PDF 13979 kb)
Rights and permissions
About this article
Cite this article
International Genetics of Ankylosing Spondylitis Consortium (IGAS). Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 45, 730–738 (2013). https://doi.org/10.1038/ng.2667
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2667
This article is cited by
-
Investigation of the acute pathogenesis of spondyloarthritis/HLA-B27-associated anterior uveitis based on genome-wide association analysis and single-cell transcriptomics
Journal of Translational Medicine (2024)
-
A study of the association between single nucleotide polymorphisms of the endoplasmic reticulum aminopeptidase 2 (ERAP2) gene and the risk of ankylosing spondylitis in Egyptians
Molecular Biology Reports (2024)
-
Role of pH-sensing receptors in colitis
Pflügers Archiv - European Journal of Physiology (2024)
-
New genomic insights into the conformation of Lipizzan horses
Scientific Reports (2023)
-
Single-Cell Analysis of Patients with Axial Spondyloarthritis After Anti-TNFα Treatment: Experimental Data and Review of the Literature
Clinical Reviews in Allergy & Immunology (2023)