Abstract
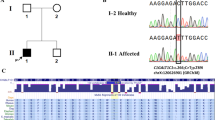
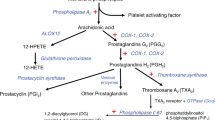
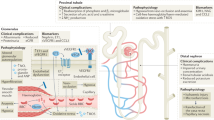
Pathologic thrombosis is a major cause of mortality. Hemolytic-uremic syndrome (HUS) features episodes of small-vessel thrombosis resulting in microangiopathic hemolytic anemia, thrombocytopenia and renal failure1. Atypical HUS (aHUS) can result from genetic or autoimmune factors2 that lead to pathologic complement cascade activation3. Using exome sequencing, we identified recessive mutations in DGKE (encoding diacylglycerol kinase ɛ) that co-segregated with aHUS in nine unrelated kindreds, defining a distinctive Mendelian disease. Affected individuals present with aHUS before age 1 year, have persistent hypertension, hematuria and proteinuria (sometimes in the nephrotic range), and develop chronic kidney disease with age. DGKE is found in endothelium, platelets and podocytes. Arachidonic acid–containing diacylglycerols (DAG) activate protein kinase C (PKC), which promotes thrombosis, and DGKE normally inactivates DAG signaling. We infer that loss of DGKE function results in a prothrombotic state. These findings identify a new mechanism of pathologic thrombosis and kidney failure and have immediate implications for treating individuals with aHUS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
NCBI Reference Sequence
Referenced accessions
NCBI Reference Sequence
References
Neild, G.H. Haemolytic-uraemic syndrome in practice. Lancet 343, 398–401 (1994).
Loirat, C. & Frémeaux-Bacchi, V. Atypical hemolytic uremic syndrome. Orphanet J. Rare Dis. 6, 60 (2011).
Noris, M., Mescia, F. & Remuzzi, G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 8, 622–633 (2012).
Noris, M. & Remuzzi, G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 1676–1687 (2009).
Zuber, J., Fakhouri, F., Roumenina, L.T., Loirat, C. & Frémeaux-Bacchi, V. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat. Rev. Nephrol. 8, 643–657 (2012).
Tang, W., Bunting, M., Zimmerman, G.A., McIntyre, T.M. & Prescott, S.M. Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. J. Biol. Chem. 271, 10237–10241 (1996).
Shulga, Y.V., Topham, M.K. & Epand, R.M. Regulation and functions of diacylglycerol kinases. Chem. Rev. 111, 6186–6208 (2011).
Rhee, S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70, 281–312 (2001).
Pettitt, T.R. et al. Diacylglycerol and phosphatidate generated by phospholipases C and D, respectively, have distinct fatty acid compositions and functions. Phospholipase D–derived diacylglycerol does not activate protein kinase C in porcine aortic endothelial cells. J. Biol. Chem. 272, 17354–17359 (1997).
Carew, M.A., Paleolog, E.M. & Pearson, J.D. The roles of protein kinase C and intracellular Ca2+ in the secretion of von Willebrand factor from human vascular endothelial cells. Biochem. J. 286, 631–635 (1992).
Ren, S., Shatadal, S. & Shen, G.X. Protein kinase C-β mediates lipoprotein-induced generation of PAI-1 from vascular endothelial cells. Am. J. Physiol. Endocrinol. Metab. 278, E656–E662 (2000).
Whatley, R.E. et al. The regulation of platelet-activating factor production in endothelial cells. The role of calcium and protein kinase C. J. Biol. Chem. 264, 6325–6333 (1989).
Herbert, J.M., Savi, P., Laplace, M.C., Dumas, A. & Dol, F. Chelerythrine, a selective protein kinase C inhibitor, counteracts pyrogen-induced expression of tissue factor without effect on thrombomodulin down-regulation in endothelial cells. Thromb. Res. 71, 487–493 (1993).
Levin, E.G., Marotti, K.R. & Santell, L. Protein kinase C and the stimulation of tissue plasminogen activator release from human endothelial cells. Dependence on the elevation of messenger RNA. J. Biol. Chem. 264, 16030–16036 (1989).
Offermans, S. Activation of platelet function through G protein−coupled receptors. Circ. Res. 15, 1293–1304 (2006).
Pettitt, T.R. & Wakelam, M.J. Diacylglycerol kinase ɛ, but not ζ, selectively removes polyunsaturated diacylglycerol, inducing altered protein kinase C distribution in vivo. J. Biol. Chem. 274, 36181–36186 (1999).
Yada, Y., Ozeki, T., Kanoh, H. & Nozawa, Y. Purification and characterization of cytosolic diacylglycerol kinases of human platelets. J. Biol. Chem. 265, 19237–19243 (1990).
Nunn, D.L. & Watson, S.P. A diacylglycerol kinase inhibitor, R59022, potentiates secretion by and aggregation of thrombin-stimulated human platelets. Biochem. J. 243, 809–813 (1987).
de Nucci, G., Gryglewski, R.J., Warner, T.D. & Vane, J.R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc. Natl. Acad. Sci. USA 85, 2334–2338 (1988).
Hofmann, T. et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 (1999).
Quack, I. et al. PKC mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia. J. Biol. Chem. 286, 12959–12970 (2011).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Sison, K. et al. Glomerular structure and function require paracrine, not autocrine, VEGF–VEGFR-2 signaling. J. Am. Soc. Nephrol. 21, 1691–1701 (2010).
Hoshi, S., Nomoto, K., Kuromitsu, J., Tomari, S. & Nagata, M. High glucose induced VEGF expression via PKC and ERK in glomerular podocytes. Biochem. Biophys. Res. Commun. 290, 177–184 (2002).
Rask-Madsen, C. & King, G.L. Differential regulation of VEGF signaling by PKC-α and PKC-ɛ in endothelial cells. Arterioscl. Throm. Vasc. Biol. 28, 919–924 (2008).
Rodriguez de Turco, E.B. et al. Diacylglycerol kinase ɛ regulates seizure susceptibility and long-term potentiation through arachidonoyl-inositol lipid signaling. Proc. Natl. Acad. Sci. USA 98, 4740–4745 (2001).
Lansigan, F., Isufi, I. & Tagoe, C.E. Microangiopathic haemolytic anaemia resembling thrombotic thrombocytopenic purpura in systemic lupus erythematosus: the role of ADAMTS13. Rheumatology 50, 824–829 (2011).
Ganesan, C. & Maynard, S.E. Acute kidney injury in pregnancy: the thrombotic microangiopathies. J. Nephrol. 24, 554–563 (2011).
Skerka, C. et al. Autoimmune forms of thrombotic micorangiopathy and membranoproliferative glomerulonephritis: indications for a disease spectrum and common pathogenic principles. Mol. Immunol. 46, 2801–2807 (2009).
Ozaltin, F. et al. DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. J. Am. Soc. Nephrol. 24, 377–384 (2013).
Nørholm, M.H.H., Shulga, Y.V., Aoki, S., Epand, R.M. & von Heijne, G. Flanking residues help determine whether a hydrophobic segment adopts a monotopic or bitopic topology in the endoplasmic reticulum membrane. J. Biol. Chem. 286, 25284–25290 (2011).
Soldin, S.J., Brugnara, C. & Wong, E.C. (eds). Pediatric Reference Intervals (AACC Press, Washington, DC, 2005).
Sellier-Leclerc, A.-L. et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 18, 2392–2400 (2007).
Frémeaux-Bacchi, V. et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin. J. Am. Soc. Nephrol. published online, http://dx.doi.org/10.2215/CJN.04760512 (2013).
Roumenina, L.T. et al. Alternative complement pathway assessment in patients with atypical HUS. J. Immunol. Methods 365, 8–26 (2011).
Boyden, L.M. et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482, 98–102 (2012).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Barrett, J.C., Fry, B., Maller, J. & Daly, M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Reeve, J.P. & Rannala, B. DMLE+: Bayesian linkage disequilibrium gene mapping. Bioinformatics 18, 894–895 (2002).
Genin, E., Tullio-Pelet, A., Begeot, F., Lyonnet, S. & Abel, L. Estimating the age of rare disease mutations: the example of Triple-A syndrome. J. Med. Genet. 41, 445–449 (2004).
Gudbjartsson, D.F., Jonasson, K., Frigge, M.L. & Kong, A. Allegro, a new computer program for multipoint linkage analysis. Nat. Genet. 25, 12–13 (2000).
Thiele, H. & Nürnberg, P. HaploPainter: a tool for drawing pedigrees with complex haplotypes. Bioinformatics 21, 1730–1732 (2005).
Tang, W.H. et al. Glucose and collagen regulate human platelet activity through aldose reductase induction of thromboxane. J. Clin. Invest. 121, 4462–4476 (2011).
McLean, I.W. & Nakane, P.K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J. Histochem. Cytochem. 22, 1077–1083 (1974).
Acknowledgements
We thank the subjects with aHUS, their families and the health care professionals whose participation made this study possible; J. Zhang, C. Nelson-Williams, S. Mentone, D. Beury and other members of the complement laboratory at Hôpital Européen Georges-Pompidou for technical support; the staff of the Yale Center for Genome Analysis for exome sequencing; S. Ishibe, S. Shibata, U. Scholl, M.-A. Dragon-Durey, L. Roumenina, M. Malina, Q. Vincent and L. Abel for helpful discussions; and D. Damotte (Assistance Publique-Hôpitaux de Paris, Hôpital Hôtel-Dieu, Service d'Anatomie Pathologique) for the anti-CD34 staining. This work was supported by US National Institutes of Health (NIH) grants U54 HG006504 01 (Yale Center for Mendelian Genomics), P30 DK079310 05 (Yale O'Brien Center for Kidney Research) and UL1TR00142 07 (Yale Center for Translational Science Award), grants from the Délégation Régionale à la Recherche Clinique, Assistance Publique–Hôpitaux de Paris to V.F.-B., such as Programme Hospitalier de Recherche Clinique (AOM08198) and the Association pour l'Information et la Recherche dans les maladies Rénales génétiques (AIRG France), and a European Community FP7 Grant 2012-305608 (EURenOmics) to F.S. and V.F.-B. M.L. is the recipient of a Kidney Research Scientist Core Education and National Training (KRESCENT) Program Post-Doctoral Fellowship Award from the Kidney Foundation of Canada and is a member of the Investigative Medicine PhD program at Yale University School of Medicine.
Author information
Authors and Affiliations
Contributions
M.L., V.F.-B. and R.P.L. designed experiments and analyzed data. M.C. and R.P.L. developed the exome analysis protocol. S.M.M., J.D.O., J.A. and H.T. directed the exome capture, DNA sequencing infrastructure and information technology. M.C., M.L., R.P.L., G.N. and P.N. performed bioinformatic and statistical analyses. M.L., W.J. and R.P.L. analyzed the age of shared mutation. M.L., W.J. and V.F.-B. performed Sanger sequencing. W.H.T., J.H. and F.F. performed protein blotting experiments. M.L. performed the immunofluorescence studies. M.L.Q. and F.F. performed the immunohistochemistry studies on human kidneys. F.S., S.T., F.N., F.M., D.M., G.D., V.B., B.L., L.C., M.A.M., E.S. and C.L. ascertained and evaluated patients with aHUS. C.L., V.F.-B. and F.S. recruited patients with aHUS. N.R.-L., G.W.M. and M.C.G. provided renal pathology expertise. M.L., V.F.-B. and R.P.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
F.S., F.F., C.L. and V.F.-B. have received fees from Alexion Pharmaceuticals for invited lectures and are members of an expert board supported by Alexion Pharmaceuticals. C.L. is an unpaid coordinator for France for the clinical trial 'Eculizumab in atypical HUS'. P.N. is a founder, CEO and shareholder of ATLAS Biolabs GmbH, a service provider for genomic analyses.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1–5 (PDF 8179 kb)
Rights and permissions
About this article
Cite this article
Lemaire, M., Frémeaux-Bacchi, V., Schaefer, F. et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 45, 531–536 (2013). https://doi.org/10.1038/ng.2590
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2590
This article is cited by
-
X-linked C1GALT1C1 mutation causes atypical hemolytic uremic syndrome
European Journal of Human Genetics (2023)
-
Clinical features and outcomes of patients with diacylglycerol kinase epsilon nephropathy: a nationwide experience
Pediatric Nephrology (2023)
-
Pharmacological Management of Atypical Hemolytic Uremic Syndrome in Pediatric Patients: Current and Future
Pediatric Drugs (2023)
-
Atypical severe early-onset nephrotic syndrome: Answers
Pediatric Nephrology (2022)
-
Thrombotic microangiopathy in children
Pediatric Nephrology (2022)