Abstract
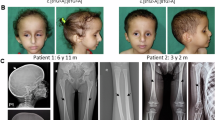
The phosphatidylinositol 3-kinase (PI3K)-AKT signaling pathway is critical for cellular growth and metabolism. Correspondingly, loss of function of PTEN, a negative regulator of PI3K, or activating mutations in AKT1, AKT2 or AKT3 have been found in distinct disorders featuring overgrowth or hypoglycemia. We performed exome sequencing of DNA from unaffected and affected cells from an individual with an unclassified syndrome of congenital progressive segmental overgrowth of fibrous and adipose tissue and bone and identified the cancer-associated mutation encoding p.His1047Leu in PIK3CA, the gene that encodes the p110α catalytic subunit of PI3K, only in affected cells. Sequencing of PIK3CA in ten additional individuals with overlapping syndromes identified either the p.His1047Leu alteration or a second cancer-associated alteration, p.His1047Arg, in nine cases. Affected dermal fibroblasts showed enhanced basal and epidermal growth factor (EGF)-stimulated phosphatidylinositol 3,4,5-trisphosphate (PIP3) generation and concomitant activation of downstream signaling relative to their unaffected counterparts. Our findings characterize a distinct overgrowth syndrome, biochemically demonstrate activation of PI3K signaling and thereby identify a rational therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Yuan, T.L. & Cantley, L.C. PI3K pathway alterations in cancer: variations on a theme. Oncogene 27, 5497–5510 (2008).
Happle, R. The group of epidermal nevus syndromes Part I. Well defined phenotypes. J. Am. Acad. Dermatol. 63, 1–22, quiz 23–24 (2010).
Lindhurst, M.J. et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N. Engl. J. Med. 365, 611–619 (2011).
Carpten, J.D. et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448, 439–444 (2007).
Happle, R. Type 2 segmental Cowden disease vs. Proteus syndrome. Br. J. Dermatol. 156, 1089–1090 (2007).
Biesecker, L. The challenges of Proteus syndrome: diagnosis and management. Eur. J. Hum. Genet. 14, 1151–1157 (2006).
Oduber, C.E., van der Horst, C.M. & Hennekam, R.C. Klippel-Trenaunay syndrome: diagnostic criteria and hypothesis on etiology. Ann. Plast. Surg. 60, 217–223 (2008).
Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 (2004).
1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Clark, J. et al. Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nat. Methods 8, 267–272 (2011).
Mandelker, D. et al. A frequent kinase domain mutation that changes the interaction between PI3Kα and the membrane. Proc. Natl. Acad. Sci. USA 106, 16996–17001 (2009).
Hon, W.C., Berndt, A. & Williams, R.L. Regulation of lipid binding underlies the activation mechanism of class IA PI3-kinases. Oncogene published online, 10.1038/onc.2011.532 (28 November 2011).
Biesecker, L.G. et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am. J. Med. Genet. 84, 389–395 (1999).
Carty, M.J., Taghinia, A. & Upton, J. Overgrowth conditions: a diagnostic and therapeutic conundrum. Hand Clin. 25, 229–245 (2009).
Oduber, C.E. et al. A proposal for classification of entities combining vascular malformations and deregulated growth. Eur. J. Med. Genet. 54, 262–271 (2011).
Chen, W.S. et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15, 2203–2208 (2001).
Whiteman, E.L., Cho, H. & Birnbaum, M.J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13, 444–451 (2002).
Hussain, K. et al. An activating mutation of AKT2 and human hypoglycemia. Science 334, 474 (2011).
Poduri, A. et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron 74, 41–48 (2012).
Hawkins, P.T., Anderson, K.E., Davidson, K. & Stephens, L.R. Signalling through Class I PI3Ks in mammalian cells. Biochem. Soc. Trans. 34, 647–662 (2006).
Yamamoto, S. et al. PIK3CA mutation is an early event in the development of endometriosis-associated ovarian clear cell adenocarcinoma. J. Pathol. 225, 189–194 (2011).
Miron, A. et al. PIK3CA mutations in in situ and invasive breast carcinomas. Cancer Res. 70, 5674–5678 (2010).
Engelman, J.A. et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat. Med. 14, 1351–1356 (2008).
Adams, J.R. et al. Cooperation between Pik3ca and p53 mutations in mouse mammary tumor formation. Cancer Res. 71, 2706–2717 (2011).
Meyer, D.S. et al. Luminal expression of PIK3CA mutant H1047R in the mammary gland induces heterogeneous tumors. Cancer Res. 71, 4344–4351 (2011).
Liu, P. et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway–dependent and PI3K pathway–independent mechanisms. Nat. Med. 17, 1116–1120 (2011).
Kinross, K.M. et al. An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. J. Clin. Invest. 122, 553–557 (2012).
Hafner, C. et al. Oncogenic PIK3CA mutations occur in epidermal nevi and seborrheic keratoses with a characteristic mutation pattern. Proc. Natl. Acad. Sci. USA 104, 13450–13454 (2007).
Tosi, L.L., Sapp, J.C., Allen, E.S., O'Keefe, R.J. & Biesecker, L.G. Assessment and management of the orthopedic and other complications of Proteus syndrome. J. Child. Orthop. 5, 319–327 (2011).
Marsh, D.J. et al. Rapamycin treatment for a child with germline PTEN mutation. Nat. Clin. Pract. Oncol. 5, 357–361 (2008).
Ellis, H., Logan, B.M. & Dixon, A.K. Human Sectional Anatomy: Atlas of Body Sections, CT and MRI Images 163 (Hodder-Arnold, London, 2007).
Raffan, E. & Semple, R.K. Next generation sequencing—implications for clinical practice. Br. Med. Bull. 99, 53–71 (2011).
Firmann, M. et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc. Disord. 8, 6 (2008).
Acknowledgements
The authors thank L. Ivey, J.J. Johnston, V. Tasic, M. Walters and E. Choolun for support and advice. The authors are especially grateful to the subjects who participated in this research study and to the Proteus Syndrome Foundations of the United States and United Kingdom, who have supported and encouraged these individuals and our research efforts. V.E.R.P., S.O., D.B.S., I.B. and R.K.S. were supported by the Wellcome Trust (grants 097721/Z/11/Z, 80952/Z/06/Z, 078986/Z/06/Z, 098051/Z/05/Z and 091551/Z/10/Z), the UK Medical Research Council Centre for Obesity and Related Disorders and the UK National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre. L.G.B., M.J.L., J.C.S. and A.M.W. were supported by the Intramural Research Program of the National Human Genome Research Institute. S.R., Q.Z. and M.J.O.W. were supported by the Biotechnology and Biological Sciences Research Council (BBSRC). We are grateful for access to exome sequence data from the CoLaus cohort, which was sequenced as part of a partnership between the Wellcome Trust Sanger Institute, the CoLaus principal investigators and the Quantitative Sciences department of GlaxoSmithKline. S.M.H. is supported by the Manchester NIHR Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
M.J.L., V.E.R.P., I.B., L.G.B. and R.K.S. designed the study and drafted the manuscript. M.J.L. and V.E.R.P. performed the research and analyzed the data. F.P., C.E.S., A.D. and I.B. performed analysis of whole-exome sequencing data and wrote the corresponding methods in the manuscript. J.C.S. provided clinical data and wrote the corresponding clinical descriptions. A.M.W. performed mutation assays and assisted with immunoblotting experiments. S.M.H., L.L.T., M.L.C., T.N.D., J.G., Z.G., V.R.S. and C.T. provided clinical data and/or fibroblast samples from affected individuals. S.R., Q.Z. and M.J.O.W. performed PIP3 quantification and data analysis. J.H. assisted with the collection of clinical data. M.P.G. assisted with immunoblotting experiments. T.H. performed histological analysis. A.K.D. interpreted imaging. S.O. and D.B.S. contributed to study design and reviewed the manuscript. L.G.B. and R.K.S. supervised the overall study.
Corresponding authors
Ethics declarations
Competing interests
I.B. and her spouse own stock in GlaxoSmithKline and the Incyte Corporation.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–3, Supplementary Figures 1 and 2 and Supplementary Note (PDF 241 kb)
Rights and permissions
About this article
Cite this article
Lindhurst, M., Parker, V., Payne, F. et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet 44, 928–933 (2012). https://doi.org/10.1038/ng.2332
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2332