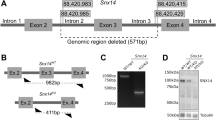
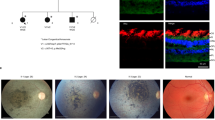
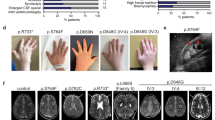
Abstract
Walker-Warburg syndrome (WWS) is an autosomal recessive multisystem disorder characterized by complex eye and brain abnormalities with congenital muscular dystrophy (CMD) and aberrant α-dystroglycan glycosylation. Here we report mutations in the ISPD gene (encoding isoprenoid synthase domain containing) as the second most common cause of WWS. Bacterial IspD is a nucleotidyl transferase belonging to a large glycosyltransferase family, but the role of the orthologous protein in chordates is obscure to date, as this phylum does not have the corresponding non-mevalonate isoprenoid biosynthesis pathway. Knockdown of ispd in zebrafish recapitulates the human WWS phenotype with hydrocephalus, reduced eye size, muscle degeneration and hypoglycosylated α-dystroglycan. These results implicate ISPD in α-dystroglycan glycosylation in maintaining sarcolemma integrity in vertebrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
van Reeuwijk, J., Brunner, H.G. & van Bokhoven, H. Glyc-O-genetics of Walker-Warburg syndrome. Clin. Genet. 67, 281–289 (2005).
Kobayashi, K. et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 394, 388–392 (1998).
Yoshida, A. et al. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev. Cell 1, 717–724 (2001).
Beltrán-Valero de Bernabé, D. et al. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am. J. Hum. Genet. 71, 1033–1043 (2002).
Brockington, M. et al. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin α2 deficiency and abnormal glycosylation of α-dystroglycan. Am. J. Hum. Genet. 69, 1198–1209 (2001).
Longman, C. et al. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of α-dystroglycan. Hum. Mol. Genet. 12, 2853–2861 (2003).
Beltrán-Valero de Bernabé, D. et al. Mutations in the FKRP gene can cause muscle-eye-brain disease and Walker-Warburg syndrome. J. Med. Genet. 41, e61 (2004).
van Reeuwijk, J. et al. POMT2 mutations cause α-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J. Med. Genet. 42, 907–912 (2005).
van Reeuwijk, J. et al. Intragenic deletion in the LARGE gene causes Walker-Warburg syndrome. Hum. Genet. 121, 685–690 (2007).
Silan, F. et al. A new mutation of the fukutin gene in a non-Japanese patient. Ann. Neurol. 53, 392–396 (2003).
Hara, Y. et al. A dystroglycan mutation associated with limb-girdle muscular dystrophy. N. Engl. J. Med. 364, 939–946 (2011).
Lefeber, D.J. et al. Deficiency of Dol-P-Man synthase subunit DPM3 bridges the congenital disorders of glycosylation with the dystroglycanopathies. Am. J. Hum. Genet. 85, 76–86 (2009).
Godfrey, C. et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain 130, 2725–2735 (2007).
Jimenez-Mallebrera, C. et al. A comparative study of α-dystroglycan glycosylation in dystroglycanopathies suggests that the hypoglycosylation of α-dystroglycan does not consistently correlate with clinical severity. Brain Pathol. 19, 596–611 (2009).
Lin, Y.Y. et al. Zebrafish Fukutin family proteins link the unfolded protein response with dystroglycanopathies. Hum. Mol. Genet. 20, 1763–1775 (2011).
Zhang, Z., Zhang, P. & Hu, H. LARGE expression augments the glycosylation of glycoproteins in addition to α-dystroglycan conferring laminin binding. PLoS ONE 6, e19080 (2011).
Bleckmann, C. et al. O-glycosylation pattern of CD24 from mouse brain. Biol. Chem. 390, 627–645 (2009).
Gilissen, C. et al. Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. Am. J. Hum. Genet. 87, 418–423 (2010).
Liu, J. & Mushegian, A. Three monophyletic superfamilies account for the majority of the known glycosyltransferases. Protein Sci. 12, 1418–1431 (2003).
Michele, D.E. et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418, 417–422 (2002).
Snow, C.J. et al. Time-lapse analysis and mathematical characterization elucidate novel mechanisms underlying muscle morphogenesis. PLoS Genet. 4, e1000219 (2008).
Parsons, M.J. et al. Zebrafish mutants identify an essential role for laminins in notochord formation. Development 129, 3137–3146 (2002).
Bassett, D.I. et al. Dystrophin is required for the formation of stable muscle attachments in the zebrafish embryo. Development 130, 5851–5860 (2003).
Straub, V., Rafael, J.A., Chamberlain, J.S. & Campbell, K.P. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 139, 375–385 (1997).
Richard, S.B. et al. Kinetic analysis of Escherichia coli 2-C-methyl-D-erythritol-4-phosphate cytidyltransferase, wild type and mutants, reveals roles of active site amino acids. Biochemistry 43, 12189–12197 (2004).
Baur, S., Marles-Wright, J., Buckenmaier, S., Lewis, R.J. & Vollmer, W. Synthesis of CDP-activated ribitol for teichoic acid precursors in Streptococcus pneumoniae. J. Bacteriol. 191, 1200–1210 (2009).
MacLeod, H. et al. A novel FKRP mutation in congenital muscular dystrophy disrupts the dystrophin glycoprotein complex. Neuromuscul. Disord. 17, 285–289 (2007).
Wood, A.J. et al. Abnormal vascular development in zebrafish models for fukutin and FKRP deficiency. Hum. Mol. Genet. 20, 4879–4890 (2011).
McMullan, D.J. et al. Molecular karyotyping of patients with unexplained mental retardation by SNP arrays: a multicenter study. Hum. Mutat. 30, 1082–1092 (2009).
Woods, C.G. et al. Quantification of homozygosity in consanguineous individuals with autosomal recessive disease. Am. J. Hum. Genet. 78, 889–896 (2006).
Parsons, M.J., Campos, I., Hirst, E.M. & Stemple, D.L. Removal of dystroglycan causes severe muscular dystrophy in zebrafish embryos. Development 129, 3505–3512 (2002).
Link, V., Shevchenko, A. & Heisenberg, C.P. Proteomics of early zebrafish embryos. BMC Dev. Biol. 6, 1 (2006).
Robu, M.E. et al. p53 activation by knockdown technologies. PLoS Genet. 3, e78 (2007).
Ciruna, B., Jenny, A., Lee, D., Mlodzik, M. & Schier, A.F. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439, 220–224 (2006).
Hall, T.E. et al. The zebrafish candyfloss mutant implicates extracellular matrix adhesion failure in laminin α2–deficient congenital muscular dystrophy. Proc. Natl. Acad. Sci. USA 104, 7092–7097 (2007).
Venselaar, H., Te Beek, T.A., Kuipers, R.K., Hekkelman, M.L. & Vriend, G. Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinformatics 11, 548 (2010).
Kemp, L.E., Bond, C.S. & Hunter, W.N. Structure of a tetragonal crystal form of Escherichia coli 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase. Acta Crystallogr. D Biol. Crystallogr. 59, 607–610 (2003).
Acknowledgements
We thank all family members who participated in this study. We would also like to thank A. Charon for referring an affected individual, G. Powell and S. Gerety for critical comments on the manuscript and R. Schot for study of the deletion in family WWS-161. This project was supported by the Large-Scale Integrating Project GENCODYS-Genetic and Epigenetic Networks in COgnitive DYSfunction (241995), which is funded by the European Union Framework Programme 7 (FP7) Health program (to H.v.B.), the Australian National Health and Medical Research Council (NHMRC) with an overseas postdoctoral fellowship (to T.R.), The Prinses Beatrix Fund (grant W.OR09-15 to D.L. and H.v.B.), the Hersenstichting Nederland (to H.v.B.) and a European Molecular Biology Organization (EMBO) Long-Term Fellowship (ALTF 805-2009 to K.B.). All zebrafish work was sponsored by the Wellcome Trust (grant WT 077047/Z/05/Z and WT 077037/Z/05/Z). Next-generation sequencing experiments were financially supported by the Department of Human Genetics at Nijmegen, as well as by the Netherlands Organisation for Health Research and Development (ZonMW; grant 916-86-016 to L.E.L.M.V.).
Author information
Authors and Affiliations
Contributions
The study was designed and results were interpreted by T.R., K.B., D.L.S., Y.-Y.L., H.G.B., D.J.L. and H.v.B. Subject ascertainment and recruitment were carried out by I.M., U.A., B.G., G.M.S.M., P.D., M.A.W., D.P.R., D.C., C.B., E.S., E.A.J.P., G.M.B.T.-S., C.E.d.D.-S., K.D., H.K., O.A.E.-F.E.-H. and H.v.B. Sequencing, CNV analysis and genotyping were carried out and interpreted by T.R., E.-J.K., K.B., I.M., J.v.R., C.v.d.E., E.v.B., M.R., R.P., L.E.L.M.V., M.S., M.F.B., H.Z., J.A.V., C.G., G.M.S.M. and H.v.B. Zebrafish studies were designed and carried out by K.B., D.L.S. and Y.-Y.L. Bioinformatic data analysis and protein modeling were performed by T.R., J.v.R., C.G. and D.J.L. The manuscript was drafted by T.R., K.B., D.J.L., Y.-Y.L. and H.v.B. All authors contributed to the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1 and 2 (PDF 2560 kb)
Rights and permissions
About this article
Cite this article
Roscioli, T., Kamsteeg, EJ., Buysse, K. et al. Mutations in ISPD cause Walker-Warburg syndrome and defective glycosylation of α-dystroglycan. Nat Genet 44, 581–585 (2012). https://doi.org/10.1038/ng.2253
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2253