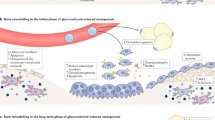
Abstract
Survival of patients with systemic lupus erythematosus (SLE) has improved over the past decade, thanks to improved treatment of the disease, which now results in fewer fatal complications. This improvement has allowed physicians to focus their attention on the prevention of organ damage caused by this chronic, inflammatory disease, and by the medications used to control the disease. Osteoporosis is common in SLE patients; risk factors for osteoporosis include prolonged use of glucocorticoids, cyclophosphamide and possibly gonadotropin-releasing-hormone agonists. In premenopausal women with SLE, inflammation or SLE-related medications can increase bone turnover, which eventually weakens bone architecture, then reduces bone strength and increases the risk of fracture. Prevention and treatment of osteoporosis in SLE patients should entail a multifaceted approach. Levels of calcium, vitamin D and homocysteine should be evaluated, and age-appropriate supplementation instituted. The bone loss that results from systemic inflammation should be treated by reduction of the inflammation with glucocorticoids, potent anti-inflammatory agents or antiresorptive agents. The efficacy of this therapy can be monitored using bone mineral density scans. This Review briefly discusses the pathophysiology of the localized and generalized osteoporosis and osteonecrosis in SLE patients and recommends therapies to both prevent and treat these unfortunate complications of this disease.
Key Points
-
Survival of patients with systemic lupus erythematosus has improved, such that treatment is now focused on minimizing the organ damage associated with either the disease itself or the medications used to treat it
-
Systemic inflammation increases osteoclast maturation and reduces osteoblast maturation and activity, such that rapid bone loss can occur
-
Compared with cortical bone, trabecular bone is more severely affected by systemic inflammation and other metabolic changes, owing to its high turnover rate
-
Patients should be carefully evaluated for bone health via measurement of bone mineral density, and laboratory evaluation of levels of calcium and vitamin D and biochemical markers of bone turnover
-
Prevention and treatment of bone loss in systemic lupus erythematosus is multifaceted and should include aggressive reduction of systemic inflammation, antiresorptive agents (if a patient has had a fracture or has a low bone mineral density), and appropriate supplementation of calcium and vitamin D
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mundy GR et al. (2003) Bone remodeling. In Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism 46–57 edn 5 (Ed Favus M). Washington DC: American Society for Bone and Mineral Research
Cooper C (2003) Epidemiology of osteoporosis. In Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism 307–313 edn 5 (Ed Favus M). Washington DC: American Society for Bone and Mineral Research
Kipen Y et al. (1997) Prevalence of reduced bone mineral density in systemic lupus erythematosus and the role of steroids. J Rheumatol 24: 1922–1929
Coimbra IB and Costallat LT (2003) Bone mineral density in systemic lupus erythematosus and its relation to age at disease onset, plasma estradiol and immunosuppressive therapy. Joint Bone Spine 70: 20–23
Mok CC et al. (2005) Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus 14: 106–112
Bultink IEM et al. (2005) Prevalence of and risk factors for low bone mineral density and vertebral fractures in patients with systemic lupus erythematosus. Arthritis Rheum 54: 2044–2050
Ramsey-Goldman R et al. (1999) Frequency of fractures in women with systemic lupus erythematosus. Arthritis Rheum 42: 882–890
Yee C-S et al. (2005) Prevalence and predictors of fragility fractures in systemic lupus erythematosus. Ann Rheum Dis 64: 111–113
Lee C and Ramsey-Goldman R (2005) Bone health and systemic lupus erythematosus. Curr Rheumatol Rep 7: 482–489
Van Staa TP et al. (2003) Bone density threshold and other predictors of vertebral fractures in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48: 224–229
Koyama H et al. (1997) Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer 39: 1403–1409
Lee H et al. (2005) Changes in bone mineral density and body composition during initial and long term gonadotropin releasing hormone agonist treatment for prostate carcinoma. Cancer 104: 1633–1637
Gambacciani M et al. (1997) Ipriflavone prevents loss of bone mass in pharmacological menopause induced by GnRH-agonists. Calcif Tissue Int 61 (Suppl): S15–S18
Svenungsson E et al. (2003) Elevated triglycerides and low levels of high-density lipoproteins as markers of disease activity in association with up-regulation of the tumor necrosis factor alpha/tumor necrosis factor receptor system in systemic lupus erythematosus. Arthritis Rheum 48: 2533–2540
Frostegard J (2005) SLE, atherosclerosis and cardiovascular disease. J Intern Med 257: 485–495
Frostegard J (2005) Atherosclerosis in patients with autoimmune disorders. Arterioscler Thomb 25: 1776–1785
Frostegard J et al. (1992) Induction of T cell activation by oxidized low density lipoprotein. Arterioscler Thromb 12: 461–467
Gabay C et al. (1997) Circulating levels of tumor necrosis factor soluble receptors in SLE are significantly higher than in other rheumatic diseases and correlate with disease activity. J Rheumatol 24: 303–308
Studnicka-Benke A et al. (1996) Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol 35: 1067–1074
Svenungsson E (2003) TNF alpha: a link between hypertriglyceridaemia and inflammation in SLE patients with cardiovascular disease. Lupus 12: 454–461
Gravallese EM and Goldring SR (2000) Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheum 43: 2143–2151
Goldring SR and Gravellese EM (2000) Mechanisms of bone loss in inflammatory arthritis: diagnosis and therapeutic implications. Arthritis Res 2: 33–37
Frostegard J (2005) Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum 52: 192–200
Takayanagi H et al. (2000) T cell mediated regulation of osteoclastogenesis by signaling cross-talk between RANKL and IFN-gamma. Nature 408: 600–605
Kotake S et al. (2001) Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum 44: 1003–1012
Kong YY et al. (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402: 304–209
Zwerina J et al. (2004) Single and combined inhibitions of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor induced arthritis: effects of synovial inflammation, bone erosion and cartilage destruction. Arthritis Rheum 50: 277–290
Kwan Tat S et al. (2004) IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev 15: 49–60
Stemme S et al. (1995) T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoproteins. Proc Natl Acad Sci USA 92: 3893–3897
Moerman EJ et al. (2004) Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMD signaling pathways. Aging Cell 3: 379–389
Ginaldi L et al. (2005) Osteoporosis, inflammation and aging. Immun Ageing 2: 14–18
Kliewer SA et al. (1998) Fatty acids and icosanoids regulate gene expression through direct interactions with peroxisome proliferators-activated receptors alpha and gamma. Proc Natl Acad Sci USA 94: 4318–4323
Davies SS et al. (2001) Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J Biol Chem 276: 16015–16023
Parhami F et al. (2001) Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res 16: 182–188
McLean RR et al. (2004) Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med 350: 2042–2049
Yesilova Z et al. (2005) Hyperhomocysteinemia in patients with Behcet's disease: is it due to inflammation or therapy? Rheumatol Int 25: 423–428
Lane NE (2001) An update on glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am 27: 235–254
Cunnane G and Lane NE (2000) Steroid-induced osteoporosis in systemic lupus erythematosus. Rheum Dis Clin North Am 26: 311–329
Manolagas SC and Weinstein RS (1999) New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res 14: 1061–1066
Weinstein RS et al. (1998) Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. J Clin Invest 102: 274–282
Johnell O et al. (2002) Oral corticosteroids increase fracture risk independently of BMD [abstract]. Osteoporosis Int 1 (Suppl): S14
Dempster D (1989) Iliac crest biopsy: bone histomorphometry in glucocorticoid-induced osteoporosis. J Bone Miner Res 4: 137–141
Rehman Q et al. (2002) Quantitative computed tomography of the lumbar spine, not dual X-ray absorptiometry, is an independent predictor of prevalent vertebral fractures in postmenopausal women with osteopenia receiving long-term glucocorticoid and hormone-replacement therapy. Arthritis Rheum 46: 1292–1297
Lian KC et al. (2005) Differences in hip quantitative computed tomography (QCT) measurements of bone mineral density and bone strength between glucocorticoid-treated and glucocorticoid-naive postmenopausal women. Osteoporosis Int 16: 642–650
Lane NE et al. (2006) A proposed mechanism for increased bone fragility in patients treated with glucocorticoids: enlargement and hypomineralization of bone matrix surrounding osteocyte lacunae. J Bone Miner Res 21: 466–476
Balooch G et al. (2005) Both risedronate and PTH maintain bone mechanical properties and matrix composition in a mouse model of glucocorticoid-induced osteoporosis. J Bone Miner Res 20 (Suppl 1): S067
Rizzo M and Urbaniak JR (2005) Osteonecrosis. In Kelley's Textbook of Rheumatology, 1812–1828 (vol 2) edn 7 (Eds Harris et al.). Philadelphia: Elsevier, Saunders
Wang GJ et al. (1997) Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am 59: 729–735
Kelman A and Lane NE (2005) The management of secondary osteoporosis. Best Pract Res Clin Rheumatol 19: 1021–1037
Watts N (2003) Biophosphates for treatment of osteoporosis. In Primer on the Metabolic Bone Disease and Disorders of Mineral Metabolism, 336–341 edn 5 (Ed Favus M). Washington DC: American Society for Bone and Mineral Research
Neer RM et al. (2001) Effect of parathyroid hormone1–34 on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344: 1434–1441
Lane NE et al. (1998) Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest 102: 1627–1633
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Lane, N. Therapy Insight: osteoporosis and osteonecrosis in systemic lupus erythematosus. Nat Rev Rheumatol 2, 562–569 (2006). https://doi.org/10.1038/ncprheum0298
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0298
This article is cited by
-
Prevention and Treatment of Bone Disease in Systemic Lupus Erythematosus
Current Treatment Options in Rheumatology (2016)
-
Symptomatic knee osteonecrosis in patients with systemic lupus erythematosus: a case–control study
Rheumatology International (2016)
-
Transplantation of mesenchymal stem cells ameliorates secondary osteoporosis through interleukin-17-impaired functions of recipient bone marrow mesenchymal stem cells in MRL/lpr mice
Stem Cell Research & Therapy (2015)
-
Lower P1NP serum levels: a predictive marker of bone loss after 1 year follow-up in premenopausal systemic lupus erythematosus patients
Osteoporosis International (2015)
-
RANKL and OPG gene polymorphisms: associations with vertebral fractures and bone mineral density in premenopausal systemic lupus erythematosus
Osteoporosis International (2015)