Abstract
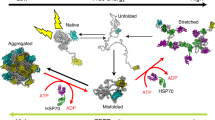
Hsp70-Hsp40-NEF and possibly Hsp100 are the only known molecular chaperones that can use the energy of ATP to convert stably pre-aggregated polypeptides into natively refolded proteins. However, the kinetic parameters and ATP costs have remained elusive because refolding reactions have only been successful with a molar excess of chaperones over their polypeptide substrates. Here we describe a stable, misfolded luciferase species that can be efficiently renatured by substoichiometric amounts of bacterial Hsp70-Hsp40-NEF. The reactivation rates increased with substrate concentration and followed saturation kinetics, thus allowing the determination of apparent Vmax′ and Km′ values for a chaperone-mediated renaturation reaction for the first time. Under the in vitro conditions used, one Hsp70 molecule consumed five ATPs to effectively unfold a single misfolded protein into an intermediate that, upon chaperone dissociation, spontaneously refolded to the native state, a process with an ATP cost a thousand times lower than expected for protein degradation and resynthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Anfinsen, C.B. Principles that govern folding of protein chains. Science 181, 223–230 (1973).
Dobson, C.M. Protein folding and misfolding. Nature 426, 884–890 (2003).
Parsell, D.A., Kowal, A.S., Singer, M.A. & Lindquist, S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372, 475–478 (1994).
Wiech, H., Buchner, J., Zimmermann, R. & Jakob, U. Hsp90 chaperones protein folding in vitro. Nature 358, 169–170 (1992).
Schröder, H., Langer, T., Hartl, F.U. & Bukau, B. DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J. 12, 4137–4144 (1993).
Cheng, M.Y. et al. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature 337, 620–625 (1989).
Goloubinoff, P., Christeller, J.T., Gatenby, A.A. & Lorimer, G.H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfolded state depends on two chaperonin proteins and Mg-ATP. Nature 342, 884–889 (1989).
Jakob, U., Gaestel, M., Engel, K. & Buchner, J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 268, 1517–1520 (1993).
Fenton, W.A., Kashi, Y., Furtak, K. & Horwich, A.L. Residues in chaperonin GroEL required for polypeptide binding and release. Nature 371, 614–619 (1994).
Rüdiger, S., Germeroth, L., Schneider-Mergener, J. & Bukau, B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16, 1501–1507 (1997).
Rüdiger, S., Schneider-Mergener, J. & Bukau, B. Its substrate specificity characterizes the DnaJ co-chaperone as a scanning factor for the DnaK chaperone. EMBO J. 20, 1042–1050 (2001).
Hartl, F.U. & Hayer-Hartl, M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295, 1852–1858 (2002).
Hinault, M.P., Ben-Zvi, A. & Goloubinoff, P. Chaperones and proteases:cellular fold-controlling factors of proteins in neurodegenerative diseases and aging. J. Mol. Neurosci. 30, 249–265 (2006).
Azem, A., Diamant, S., Kessel, M., Weiss, C. & Goloubinoff, P. The protein—folding activity of chaperonins correlates with the symmetric GroEL14(GroES7)2 heterooligomer. Proc. Natl. Acad. Sci. USA 92, 12021–12025 (1995).
Diamant, S., Azem, A., Weiss, C. & Goloubinoff, P. Effect of free and ATP-bound magnesium and manganese ions on the ATPase activity of chaperonin GroEL14. Biochemistry 34, 273–277 (1995).
Martin, J. et al. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature 352, 36–42 (1991).
Ben-Zvi, A., De los Rios, P., Dietler, G. & Goloubinoff, P. Active solubilization and refolding of stable protein aggregates by cooperative unfolding action of individual Hsp70 chaperones. J. Biol. Chem. 279, 37298–37303 (2004).
Diamant, S., Ben-Zvi, A.P., Bukau, B. & Goloubinoff, P. Size-dependent disaggregation of stable protein aggregates by the DnaK chaperone machinery. J. Biol. Chem. 275, 21107–21113 (2000).
Diamant, S. & Goloubinoff, P. Temperature-controlled activity of DnaK-DnaJ-GrpE chaperones: Protein-folding arrest and recovery during and after heat shock depends on the substrate protein and the GrpE concentration. Biochemistry 37, 9688–9694 (1998).
Skowyra, D., Georgopoulos, C. & Zylicz, M. The E. coli dnaK gene product, the Hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell 62, 939–944 (1990).
Veinger, L., Diamant, S., Buchner, J. & Goloubinoff, P. The small heat-shock protein IbpB from Escherichia coli stabilizes stress-denatured proteins for subsequent refolding by a multichaperone network. J. Biol. Chem. 273, 11032–11037 (1998).
Schuermann, J.P. et al. Structure of the Hsp110: Hsc70 nucleotide exchange machine. Mol. Cell 31, 232–243 (2008).
Glover, J.R. & Lindquist, S. Hsp104, Hsp70, and Hsp40: A novel chaperone system that rescues previously aggregated proteins. Cell 94, 73–82 (1998).
Goloubinoff, P., Mogk, A., Ben Zvi, A.P., Tomoyasu, T. & Bukau, B. Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc. Natl. Acad. Sci. USA 96, 13732–13737 (1999).
Szabo, A. et al. The ATP hydrolysis-dependent reaction cycle of the Escherichia coli Hsp70 system DnaK, DnaJ, and GrpE. Proc. Natl. Acad. Sci. USA 91, 10345–10349 (1994).
Diamant, S., Azem, A., Weiss, C. & Goloubinoff, P. Increased efficiency of GroE-assisted protein-folding by manganese ions. J. Biol. Chem. 270, 28387–28391 (1995).
Sharma, S.K., Christen, P. & Goloubinoff, P. Disaggregating chaperones: An unfolding story. Curr. Protein Pept. Sci. 10, 432–446 (2009).
De Los Rios, P., Ben-Zvi, A., Slutsky, O., Azem, A. & Goloubinoff, P. Hsp70 chaperones accelerate protein translocation and the unfolding of stable protein aggregates by entropic pulling. Proc. Natl. Acad. Sci. USA 103, 6166–6171 (2006).
Svetlov, M.S., Kolb, V.A. & Spirin, A.S. Folding of the firefly luciferase polypeptide chain with the immobilized C terminus. Mol. Biol. 41, 86–92 (2007).
Conti, E., Franks, N.P. & Brick, P. Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure 4, 287–298 (1996).
Fishbein, W.N. & Winkert, J.W. in Proteins at Low Temperatures, Vol. 180 (ed. Fennema, O.) 55–82 (American Chemical Society, 1979).
Barouch, W., Prasad, K., Greene, L. & Eisenberg, E. Auxilin-induced interaction of the molecular chaperone Hsc70 with clathrin baskets. Biochemistry 36, 4303–4308 (1997).
Han, W. & Christen, P. Mechanism of the targeting action of DnaJ in the DnaK molecular chaperone system. J. Biol. Chem. 278, 19038–19043 (2003).
Laufen, T. et al. Mechanism of regulation of Hsp70 chaperones by DnaJ cochaperones. Proc. Natl. Acad. Sci. USA 96, 5452–5457 (1999).
Brehmer, D., Gassler, C., Rist, W., Mayer, M.P. & Bukau, B. Influence of GrpE on DnaK-substrate interactions. J. Biol. Chem. 279, 27957–27964 (2004).
Mally, A. & Witt, S.N. GrpE accelerates peptide binding and release from the high affinity state of DnaK. Nat. Struct. Biol. 8, 254–257 (2001).
Hu, B., Mayer, M.P. & Tomita, M. Modeling Hsp70-mediated protein folding. Biophys. J. 91, 496–507 (2006).
Mayer, M.P. et al. Multistep mechanism of substrate binding determines chaperone activity of Hsp70. Nat. Struct. Biol. 7, 586–593 (2000).
Popp, S. et al. Structural dynamics of the DnaK-peptide complex. J. Mol. Biol. 347, 1039–1052 (2005).
Bertelsen, E.B., Chang, L., Gestwicki, J.E. & Zuiderweg, E.R. Solution conformation of wild-type E. coli Hsp70 (DnaK) chaperone complexed with ADP and substrate. Proc. Natl. Acad. Sci. USA 106, 8471–8476 (2009).
Goloubinoff, P. & De Los Rios, P. The mechanism of Hsp70 chaperones: (entropic) pulling the models together. Trends Biochem. Sci. 32, 372–380 (2007).
Gamer, J., Bujard, H. & Bukau, B. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor sigma 32. Cell 69, 833–842 (1992).
Rodriguez, F. et al. Molecular basis for regulation of the heat shock transcription factor sigma32 by the DnaK and DnaJ chaperones. Mol. Cell 32, 347–358 (2008).
Muchowski, P.J. & Wacker, J.L. Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci. 6, 11–22 (2005).
Feifel, B., Sandmeier, E., Schonfeld, H.J. & Christen, P. Potassium ions and the molecular-chaperone activity of DnaK. Eur. J. Biochem. 237, 318–321 (1996).
Hellebust, H., Uhlen, M. & Enfors, S.O. Interaction between heat-shock protein Dnak and recombinant staphylococcal protein-A. J. Bacteriol. 172, 5030–5034 (1990).
Schönfeld, H.J., Schmidt, D., Schroder, H. & Bukau, B. The Dnak chaperone system of Escherichia coli—quaternary structures and interactions of the Dnak and Grpe components. J. Biol. Chem. 270, 2183–2189 (1995).
Bischofberger, P., Han, W.J., Feifel, B., Schonfeld, H.J. & Christen, P. D-Peptides as inhibitors of the DnaK/DnaJ/GrpE chaperone system. J. Biol. Chem. 278, 19044–19047 (2003).
Acknowledgements
We thank A.S. Spirin (Institute of Protein Research, Russian Academy of Sciences) for the luciferase plasmid, H.J. Schönfeld (F. Hoffmann-La Roche) for DnaK, DnaJ and GrpE, A. Azem (Tel Aviv University) for discussions and AUC analyses, M. Muriset and R.U.H. Mattoo for discussions and technical assistance, G. Lorimer and J. Buchner for suggesting, respectively, the experiments in Figures 5b and 2b, and A. Finka and S. Priya for discussions and manuscript correction. This research was financed by grant 3100A0-109290 from the Swiss National Science Foundation and in part by the Zwahlen Grant from the Faculty of Biology and Medicine, University of Lausanne.
Author information
Authors and Affiliations
Contributions
S.K.S. designed and executed the experiments; P.D.L.R. designed some experiments; P.C. designed and analyzed some experiments. A.L. performed the analytical ultracentrifugation; P.G. directed the project, designed the experiments, analyzed the data and wrote the paper with P.D.L.R. and P.C. All authors contributed to the final text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1–3 (PDF 590 kb)
Rights and permissions
About this article
Cite this article
Sharma, S., De Los Rios, P., Christen, P. et al. The kinetic parameters and energy cost of the Hsp70 chaperone as a polypeptide unfoldase. Nat Chem Biol 6, 914–920 (2010). https://doi.org/10.1038/nchembio.455
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.455
This article is cited by
-
Microbial gene expression in Guaymas Basin subsurface sediments responds to hydrothermal stress and energy limitation
The ISME Journal (2023)
-
A fluorescent multi-domain protein reveals the unfolding mechanism of Hsp70
Nature Chemical Biology (2023)
-
Expression of a Heat Shock Protein 70 from the Brown Alga Ectocarpus sp. Imparts Salinity Stress Tolerance in Arabidopsis thaliana
Journal of Applied Phycology (2023)
-
Promiscuous interaction of titanium dioxide nanoparticles leads to alterations in structural stability and interferes with luciferase folding
Journal of Nanoparticle Research (2023)
-
Trigger factor both holds and folds its client proteins
Nature Communications (2022)