Abstract
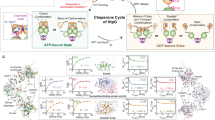
The Hsp90 chaperone is a central node of protein homeostasis, activating many diverse client proteins. Hsp90 functions as a molecular clamp that closes and opens in response to the binding and hydrolysis of ATP. Crystallographic studies have defined distinct conformational states of the mechanistic core, implying structural changes that have not yet been observed in solution. Here we engineered one-nanometer fluorescence probes based on photoinduced electron transfer into the yeast Hsp90 to observe these motions. We found that the ATPase activity of the chaperone was reflected in the kinetics of specific structural rearrangements at remote positions that acted cooperatively. Nanosecond single-molecule fluorescence fluctuation analysis uncovered that critical structural elements that undergo rearrangement were mobile on a sub-millisecond time scale. We identified a two-step mechanism for lid closure over the nucleotide-binding pocket. The activating co-chaperone Aha1 mobilized the lid of apo Hsp90, suggesting an early role in the catalytic cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Taipale, M., Jarosz, D.F. & Lindquist, S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515–528 (2010).
Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 14, 630–642 (2013).
Nagy, P.D., Wang, R.Y., Pogany, J., Hafren, A. & Makinen, K. Emerging picture of host chaperone and cyclophilin roles in RNA virus replication. Virology 411, 374–382 (2011).
Whitesell, L. & Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 5, 761–772 (2005).
Pearl, L.H., Prodromou, C. & Workman, P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem. J. 410, 439–453 (2008).
Trepel, J., Mollapour, M., Giaccone, G. & Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10, 537–549 (2010).
Prodromou, C. et al. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 90, 65–75 (1997).
Jhaveri, K. et al. Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin. Investig. Drugs 23, 611–628 (2014).
Neckers, L. & Workman, P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin. Cancer Res. 18, 64–76 (2012).
Cullinan, S.B. & Whitesell, L. Heat shock protein 90: a unique chemotherapeutic target. Semin. Oncol. 33, 457–465 (2006).
Meyer, P. et al. Structural and functional analysis of the middle segment of hsp90: implications for ATP hydrolysis and client protein and cochaperone interactions. Mol. Cell 11, 647–658 (2003).
Vaughan, C.K. et al. Structure of an Hsp90-Cdc37-Cdk4 complex. Mol. Cell 23, 697–707 (2006).
Richter, K., Muschler, P., Hainzl, O. & Buchner, J. Coordinated ATP hydrolysis by the Hsp90 dimer. J. Biol. Chem. 276, 33689–33696 (2001).
Ali, M.M. et al. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 440, 1013–1017 (2006).
Panaretou, B. et al. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 17, 4829–4836 (1998).
Prodromou, C. et al. The ATPase cycle of Hsp90 drives a molecular 'clamp' via transient dimerization of the N-terminal domains. EMBO J. 19, 4383–4392 (2000).
Maloney, A. et al. Gene and protein expression profiling of human ovarian cancer cells treated with the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 67, 3239–3253 (2007).
Roe, S.M. et al. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 42, 260–266 (1999).
Shiau, A.K., Harris, S.F., Southworth, D.R. & Agard, D.A. Structural Analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell 127, 329–340 (2006).
Pearl, L.H. & Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 75, 271–294 (2006).
Prodromou, C. The 'active life' of Hsp90 complexes. Biochim. Biophys. Acta 1823, 614–623 (2012).
Graf, C., Stankiewicz, M., Kramer, G. & Mayer, M.P. Spatially and kinetically resolved changes in the conformational dynamics of the Hsp90 chaperone machine. EMBO J. 28, 602–613 (2009).
Hessling, M., Richter, K. & Buchner, J. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat. Struct. Mol. Biol. 16, 287–293 (2009).
Mickler, M., Hessling, M., Ratzke, C., Buchner, J. & Hugel, T. The large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. Nat. Struct. Mol. Biol. 16, 281–286 (2009).
Panaretou, B. et al. Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol. Cell 10, 1307–1318 (2002).
Meyer, P. et al. Structural basis for recruitment of the ATPase activator Aha1 to the Hsp90 chaperone machinery. EMBO J. 23, 511–519 (2004).
Roe, S.M. et al. The Mechanism of Hsp90 regulation by the protein kinase-specific cochaperone p50(cdc37). Cell 116, 87–98 (2004).
Neuweiler, H. & Sauer, M. Using photoinduced charge transfer reactions to study conformational dynamics of biopolymers at the single-molecule level. Curr. Pharm. Biotechnol. 5, 285–298 (2004).
Doose, S., Neuweiler, H. & Sauer, M. Fluorescence quenching by photoinduced electron transfer: a reporter for conformational dynamics of macromolecules. ChemPhysChem 10, 1389–1398 (2009).
Neuweiler, H., Johnson, C.M. & Fersht, A.R. Direct observation of ultrafast folding and denatured state dynamics in single protein molecules. Proc. Natl. Acad. Sci. USA 106, 18569–18574 (2009).
Neuweiler, H., Banachewicz, W. & Fersht, A.R. Kinetics of chain motions within a protein-folding intermediate. Proc. Natl. Acad. Sci. USA 107, 22106–22110 (2010).
Sauer, M. & Neuweiler, H. PET-FCS: probing rapid structural fluctuations of proteins and nucleic acids by single-molecule fluorescence quenching. Methods Mol. Biol. 1076, 597–615 (2014).
Schuler, B. & Hofmann, H. Single-molecule spectroscopy of protein folding dynamics—expanding scope and timescales. Curr. Opin. Struct. Biol. 23, 36–47 (2013).
Vaiana, A.C. et al. Fluorescence quenching of dyes by tryptophan: interactions at atomic detail from combination of experiment and computer simulation. J. Am. Chem. Soc. 125, 14564–14572 (2003).
Mishra, P. & Bolon, D.N. Designed Hsp90 heterodimers reveal an asymmetric ATPase-driven mechanism in vivo. Mol. Cell 53, 344–350 (2014).
Roughley, S.D. & Hubbard, R.E. How well can fragments explore accessed chemical space? A case study from heat shock protein 90. J. Med. Chem. 54, 3989–4005 (2011).
Richter, K., Reinstein, J. & Buchner, J. N-terminal residues regulate the catalytic efficiency of the Hsp90 ATPase cycle. J. Biol. Chem. 277, 44905–44910 (2002).
Krukenberg, K.A., Förster, F., Rice, L.M., Sali, A. & Agard, D.A. Multiple conformations of E. coli Hsp90 in solution: insights into the conformational dynamics of Hsp90. Structure 16, 755–765 (2008).
Southworth, D.R. & Agard, D.A. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol. Cell 32, 631–640 (2008).
Cochran, A.G., Skelton, N.J. & Starovasnik, M.A. Tryptophan zippers: stable, monomeric beta -hairpins. Proc. Natl. Acad. Sci. USA 98, 5578–5583 (2001).
Santiveri, C.M. & Jiménez, M.A. Tryptophan residues: scarce in proteins but strong stabilizers of β-hairpin peptides. Biopolymers 94, 779–790 (2010).
Cunningham, C.N., Southworth, D.R., Krukenberg, K.A. & Agard, D.A. The conserved arginine 380 of Hsp90 is not a catalytic residue, but stabilizes the closed conformation required for ATP hydrolysis. Protein Sci. 21, 1162–1171 (2012).
McLaughlin, S.H., Ventouras, L.A., Lobbezoo, B. & Jackson, S.E. Independent ATPase activity of Hsp90 subunits creates a flexible assembly platform. J. Mol. Biol. 344, 813–826 (2004).
Wegele, H., Muschler, P., Bunck, M., Reinstein, J. & Buchner, J. Dissection of the contribution of individual domains to the ATPase mechanism of Hsp90. J. Biol. Chem. 278, 39303–39310 (2003).
Siligardi, G. et al. Co-chaperone regulation of conformational switching in the Hsp90 ATPase cycle. J. Biol. Chem. 279, 51989–51998 (2004).
Prodromou, C. & Pearl, L.H. Structure and functional relationships of Hsp90. Curr. Cancer Drug Targets 3, 301–323 (2003).
Koulov, A.V. et al. Biological and structural basis for Aha1 regulation of Hsp90 ATPase activity in maintaining proteostasis in the human disease cystic fibrosis. Mol. Biol. Cell 21, 871–884 (2010).
Gianni, S., Ivarsson, Y., Jemth, P., Brunori, M. & Travaglini-Allocatelli, C. Identification and characterization of protein folding intermediates. Biophys. Chem. 128, 105–113 (2007).
Krukenberg, K.A., Street, T.O., Lavery, L.A. & Agard, D.A. Conformational dynamics of the molecular chaperone Hsp90. Q. Rev. Biophys. 44, 229–255 (2011).
Lavery, L.A. et al. Structural asymmetry in the closed state of mitochondrial Hsp90 (TRAP1) supports a two-step ATP hydrolysis mechanism. Mol. Cell 53, 330–343 (2014).
Li, J., Richter, K., Reinstein, J. & Buchner, J. Integration of the accelerator Aha1 in the Hsp90 co-chaperone cycle. Nat. Struct. Mol. Biol. 20, 326–331 (2013).
Retzlaff, M. et al. Asymmetric activation of the hsp90 dimer by its cochaperone aha1. Mol. Cell 37, 344–354 (2010).
Ries, J., Schwarze, S., Johnson, C.M. & Neuweiler, H. Microsecond folding and domain motions of a spider silk protein structural switch. J. Am. Chem. Soc. 136, 17136–17144 (2014).
Daidone, I., Neuweiler, H., Doose, S., Sauer, M. & Smith, J.C. Hydrogen-bond driven loop-closure kinetics in unfolded polypeptide chains. PLoS Comput. Biol. 6, e1000645 (2010).
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (grant NE 1201/3-1 to H.N.) and the Wellcome Trust (Senior Investigator Award 095605/Z11/Z to L.H.P.). A.S. was supported by a grant of the German Excellence Initiative to the Graduate School of Life Sciences (University of Würzburg).
Author information
Authors and Affiliations
Contributions
A.S. designed experiments, synthesized modified protein material, performed rapid-mixing fluorescence experiments, performed ATPase assays, analyzed data, interpreted results and wrote the paper. G.B. synthesized modified protein material, performed PET-FCS experiments, analyzed data and interpreted results. D.A.H. synthesized modified protein material, performed PET-FCS experiments and analyzed data. J.S. synthesized modified protein material, performed PET-FCS experiments and analyzed data. L.H.P. interpreted results and wrote the paper. C.P. designed experiments, interpreted results and wrote the paper. H.N. conceptually designed the research, designed experiments, analyzed data, interpreted results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–4 and Supplementary Figures 1–5. (PDF 835 kb)
Rights and permissions
About this article
Cite this article
Schulze, A., Beliu, G., Helmerich, D. et al. Cooperation of local motions in the Hsp90 molecular chaperone ATPase mechanism. Nat Chem Biol 12, 628–635 (2016). https://doi.org/10.1038/nchembio.2111
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2111