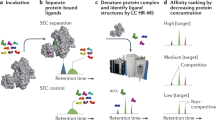
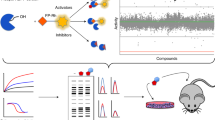
Abstract
The selectivity of an enzyme inhibitor is a key determinant of its usefulness as a tool compound or its safety as a drug. Yet selectivity is never assessed comprehensively in the early stages of the drug discovery process, and only rarely in the later stages, because technical limitations prohibit doing otherwise. Here, we report EnPlex, an efficient, high-throughput method for simultaneously assessing inhibitor potency and specificity, and pilot its application to 96 serine hydrolases. EnPlex analysis of widely used serine hydrolase inhibitors revealed numerous previously unrecognized off-target interactions, some of which may help to explain previously confounding adverse effects. In addition, EnPlex screening of a hydrolase-directed library of boronic acid– and nitrile-containing compounds provided structure-activity relationships in both potency and selectivity dimensions from which lead candidates could be more effectively prioritized. Follow-up of a series of dipeptidyl peptidase 4 inhibitors showed that EnPlex indeed predicted efficacy and safety in animal models. These results demonstrate the feasibility and value of high-throughput, superfamily-wide selectivity profiling and suggest that such profiling can be incorporated into the earliest stages of drug discovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Collins, I. & Workman, P. New approaches to molecular cancer therapeutics. Nat. Chem. Biol. 2, 689–700 (2006).
Goldstein, D.M., Gray, N.S. & Zarrinkar, P.P. High-throughput kinase profiling as a platform for drug discovery. Nat. Rev. Drug Discov. 7, 391–397 (2008).
Fabian, M.A. et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 23, 329–336 (2005).
Davis, M.I. et al. Comprehensive analysis of kinase inhibitor selectivity. Nat. Biotechnol. 29, 1046–1051 (2011).
Anastassiadis, T., Deacon, S.W., Devarajan, K., Ma, H. & Peterson, J.R. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat. Biotechnol. 29, 1039–1045 (2011).
Bachovchin, D.A. & Cravatt, B.F. The pharmacological landscape and therapeutic potential of serine hydrolases. Nat. Rev. Drug Discov. 11, 52–68 (2012).
Long, J.Z. & Cravatt, B.F. The metabolic serine hydrolases and their functions in mammalian physiology and disease. Chem. Rev. 111, 6022–6063 (2011).
Cravatt, B.F., Wright, A.T. & Kozarich, J.W. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu. Rev. Biochem. 77, 383–414 (2008).
Liu, Y., Patricelli, M.P. & Cravatt, B.F. Activity-based protein profiling: the serine hydrolases. Proc. Natl. Acad. Sci. USA 96, 14694–14699 (1999).
Leung, D., Hardouin, C., Boger, D.L. & Cravatt, B.F. Discovering potent and selective reversible inhibitors of enzymes in complex proteomes. Nat. Biotechnol. 21, 687–691 (2003).
Bachovchin, D.A., Brown, S.J., Rosen, H. & Cravatt, B.F. Identification of selective inhibitors of uncharacterized enzymes by high-throughput screening with fluorescent activity-based probes. Nat. Biotechnol. 27, 387–394 (2009).
Bachovchin, D.A. et al. Superfamily-wide portrait of serine hydrolase inhibition achieved by library-versus-library screening. Proc. Natl. Acad. Sci. USA 107, 20941–20946 (2010).
Bachovchin, D.A. et al. Academic cross-fertilization by public screening yields a remarkable class of protein phosphatase methylesterase-1 inhibitors. Proc. Natl. Acad. Sci. USA 108, 6811–6816 (2011).
Fenteany, G. et al. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268, 726–731 (1995).
Ostrowska, H., Wojcik, C., Omura, S. & Worowski, K. Lactacystin, a specific inhibitor of the proteasome, inhibits human platelet lysosomal cathepsin A–like enzyme. Biochem. Biophys. Res. Commun. 234, 729–732 (1997).
Ostrowska, H. et al. Separation of cathepsin A–like enzyme and the proteasome: evidence that lactacystin/beta-lactone is not a specific inhibitor of the proteasome. Int. J. Biochem. Cell Biol. 32, 747–757 (2000).
Fenteany, G. & Schreiber, S.L. Lactacystin, proteasome function, and cell fate. J. Biol. Chem. 273, 8545–8548 (1998).
Adams, J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell 5, 417–421 (2004).
Arastu-Kapur, S. et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin. Cancer Res. 17, 2734–2743 (2011).
Roujeau, J.C. et al. Telaprevir-related dermatitis. JAMA Dermatol. 149, 152–158 (2013).
Schlütter, J. Therapeutics: new drugs hit the target. Nature 474, S5–S7 (2011).
Talas, U., Dunlop, J., Khalaf, S., Leigh, I.M. & Kelsell, D.P. Human elastase 1: evidence for expression in the skin and the identification of a frequent frameshift polymorphism. J. Invest. Dermatol. 114, 165–170 (2000).
Tani, T., Ohsumi, J., Mita, K. & Takiguchi, Y. Identification of a novel class of elastase isozyme, human pancreatic elastase III, by cDNA and genomic gene cloning. J. Biol. Chem. 263, 1231–1239 (1988).
Sawyer, L. et al. The atomic structure of crystalline porcine pancreatic elastase at 2.5-Å resolution: comparisons with the structure of α-chymotrypsin. J. Mol. Biol. 118, 137–208 (1978).
Lindenbach, B.D. & Rice, C.M. Unravelling hepatitis C virus replication from genome to function. Nature 436, 933–938 (2005).
Liverton, N.J. et al. MK-7009, a potent and selective inhibitor of hepatitis C virus NS3/4A protease. Antimicrob. Agents Chemother. 54, 305–311 (2010).
Soisson, S.M. et al. Structural definition and substrate specificity of the S28 protease family: the crystal structure of human prolylcarboxypeptidase. BMC Struct. Biol. 10, 16 (2010).
Zhou, C. et al. Design and synthesis of prolylcarboxypeptidase (PrCP) inhibitors to validate PrCP as a potential target for obesity. J. Med. Chem. 53, 7251–7263 (2010).
Wallingford, N. et al. Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents. J. Clin. Invest. 119, 2291–2303 (2009).
Mallela, J., Yang, J. & Shariat-Madar, Z. Prolylcarboxypeptidase: a cardioprotective enzyme. Int. J. Biochem. Cell Biol. 41, 477–481 (2009).
Long, J.Z. et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc. Natl. Acad. Sci. USA 106, 20270–20275 (2009).
Munford, R.S. & Hall, C.L. Purification of acyloxyacyl hydrolase, a leukocyte enzyme that removes secondary acyl chains from bacterial lipopolysaccharides. J. Biol. Chem. 264, 15613–15619 (1989).
Long, J.Z., Jin, X., Adibekian, A., Li, W. & Cravatt, B.F. Characterization of tunable piperidine and piperazine carbamates as inhibitors of endocannabinoid hydrolases. J. Med. Chem. 53, 1830–1842 (2010).
Lu, M., Varley, A.W., Ohta, S., Hardwick, J. & Munford, R.S. Host inactivation of bacterial lipopolysaccharide prevents prolonged tolerance following Gram-negative bacterial infection. Cell Host Microbe 4, 293–302 (2008).
Lu, M. et al. Lipopolysaccharide deacylation by an endogenous lipase controls innate antibody responses to Gram-negative bacteria. Nat. Immunol. 6, 989–994 (2005).
Munford, R.S. & Erwin, A.L. Eukaryotic lipopolysaccharide deacylating enzyme. Methods Enzymol. 209, 485–492 (1992).
Perrier, J., Durand, A., Giardina, T. & Puigserver, A. Catabolism of intracellular N-terminal acetylated proteins: involvement of acylpeptide hydrolase and acylase. Biochimie 87, 673–685 (2005).
Woitach, J.T., Zhang, M., Niu, C.H. & Thorgeirsson, S.S. A retinoblastoma-binding protein that affects cell-cycle control and confers transforming ability. Nat. Genet. 19, 371–374 (1998).
Shields, D.J. et al. RBBP9: a tumor-associated serine hydrolase activity required for pancreatic neoplasia. Proc. Natl. Acad. Sci. USA 107, 2189–2194 (2010).
Bachovchin, D.A. et al. Oxime esters as selective, covalent inhibitors of the serine hydrolase retinoblastoma-binding protein 9 (RBBP9). Bioorg. Med. Chem. Lett. 20, 2254–2258 (2010).
Mentlein, R., Gallwitz, B. & Schmidt, W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 214, 829–835 (1993).
Choy, M. & Lam, S. Sitagliptin: a novel drug for the treatment of type 2 diabetes. Cardiol. Rev. 15, 264–271 (2007).
Thareja, S. et al. Saxagliptin: a new drug for the treatment of type 2 diabetes. Mini Rev. Med. Chem. 10, 759–765 (2010).
Flentke, G.R. et al. Inhibition of dipeptidyl aminopeptidase IV (DP-IV) by Xaa-boroPro dipeptides and use of these inhibitors to examine the role of DP-IV in T-cell function. Proc. Natl. Acad. Sci. USA 88, 1556–1559 (1991).
Connolly, B.A. et al. Dipeptide boronic acid inhibitors of dipeptidyl peptidase IV: determinants of potency and in vivo efficacy and safety. J. Med. Chem. 51, 6005–6013 (2008).
Lankas, G.R. et al. Dipeptidyl peptidase IV inhibition for the treatment of type 2 diabetes: potential importance of selectivity over dipeptidyl peptidases 8 and 9. Diabetes 54, 2988–2994 (2005).
Li, W., Blankman, J.L. & Cravatt, B.F. A functional proteomic strategy to discover inhibitors for uncharacterized hydrolases. J. Am. Chem. Soc. 129, 9594–9595 (2007).
Ahn, K. et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem. Biol. 16, 411–420 (2009).
Alexander, J.P. & Cravatt, B.F. Mechanism of carbamate inactivation of FAAH: implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chem. Biol. 12, 1179–1187 (2005).
Hoover, H.S., Blankman, J.L., Niessen, S. & Cravatt, B.F. Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling. Bioorg. Med. Chem. Lett. 18, 5838–5841 (2008).
Yang, X. et al. A public genome-scale lentiviral expression library of human ORFs. Nat. Methods 8, 659–661 (2011).
Martin, B.R., Giepmans, B.N., Adams, S.R. & Tsien, R.Y. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 23, 1308–1314 (2005).
Munford, R., Lu, M. & Varley, A. Chapter 2: Kill the bacteria...and also their messengers? Adv. Immunol. 103, 29–48 (2009).
Acknowledgements
We thank B. Cravatt (The Scripps Research Institute) for the FP serine hydrolase probe; B. Martin (University of Michigan), J. Long (Dana-Farber Cancer Institute), A. Lone and A. Saghatelian (both at Harvard University) for constructs; J. Davis and D. Peck (both at The Broad Institute) for technical assistance; and D. Gray and C. Yu (both at The Broad Institute) for helpful discussions. This work was supported by the National Cancer Institute (grant no. U54CA112962 to T.R.G.), Howard Hughes Medical Institute (T.R.G.), the US National Institutes of Health (NIH; grant R01 CA163930 to W.W.B.), Arisaph Pharmaceuticals (W.W.B.) and in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH (R.S.M.).
Author information
Authors and Affiliations
Contributions
D.A.B. conceived and developed the EnPlex assay, performed experiments and analyzed data; L.W.K. assisted with enzyme cloning and expression; W.W., Y. Liu, Y. Li, P.Z., I.W. and Y.S. synthesized and characterized boronic acid– and nitrile-based compounds; J.H.L. directed the synthetic efforts; S.E.P. carried out standard enzymatic substrate assays (HTRA2 and DPPs); C.P.K. directed monkey toxicity studies; S.E.H. and M.D. carried out mouse oral glucose tolerance test (OGTT) studies; D.G.S. directed OGTT studies; R.S.M. performed AOAH experiments; W.W.B. directed synthesis, enzymatic substrate assays and OGTT experiments; T.R.G. directed the project; and D.A.B. and T.R.G. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
W.B.W. is a co-founder, advisor and board member of Arisaph Pharmaceuticals, a biotechnology company interested in developing boronic acid-based inhibitors of serine hydrolases as therapeutics. C.K. is a co-founder, CEO and board member of Arisaph Pharmaceuticals.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Figures 1–11, Supplementary Tables 1–8 and Supplementary Notes 1 and 2. (PDF 14546 kb)
Supplementary Data Set
IC50 values (nM) for 55 inhibitors against 94 serine hydrolases. Blank entries indicate that the IC50 was greater than 33 mM. (XLSX 67 kb)
Rights and permissions
About this article
Cite this article
Bachovchin, D., Koblan, L., Wu, W. et al. A high-throughput, multiplexed assay for superfamily-wide profiling of enzyme activity. Nat Chem Biol 10, 656–663 (2014). https://doi.org/10.1038/nchembio.1578
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1578
This article is cited by
-
A small molecule inhibitor prevents gut bacterial genotoxin production
Nature Chemical Biology (2023)
-
The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic
Nature Reviews Endocrinology (2020)
-
High-Resolution Confocal Fluorescence Imaging of Serine Hydrolase Activity in Cryosections – Application to Glioma Brain Unveils Activity Hotspots Originating from Tumor-Associated Neutrophils
Biological Procedures Online (2020)
-
DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia
Nature Medicine (2018)
-
DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis
Nature Chemical Biology (2017)