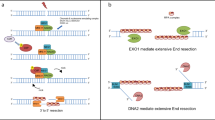
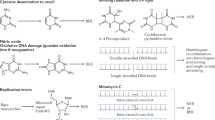
Abstract
Mutations can be beneficial under conditions in which genetic diversity is advantageous, such as somatic hypermutation and antibody generation, but they can also be lethal when they disrupt basic cellular processes or cause uncontrolled proliferation and cancer. Mutations arise from inaccurate processing of lesions generated by endogenous and exogenous DNA damaging agents, and the genome is particularly vulnerable to such damage during S phase. In this phase of the cell cycle, many lesions in the DNA template block replication. Such lesions must be bypassed in order to preserve fork stability and to ensure completion of DNA replication. Lesion bypass is carried out by a set of error-prone and error-free processes collectively referred to as DNA damage tolerance mechanisms. Here, we discuss how two types of DNA damage tolerance, translesion synthesis and template switching, are regulated at stalled replication forks by ubiquitination of PCNA, and the conditions under which they occur.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Friedberg, E.C. Suffering in silence: the tolerance of DNA damage. Nat. Rev. Mol. Cell Biol. 6, 943–953 (2005).
Cimprich, K.A. & Cortez, D. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9, 616–627 (2008).
Nyberg, K.A., Michelson, R.J., Putnam, C.W. & Weinert, T.A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36, 617–656 (2002).
Paulsen, R.D. & Cimprich, K.A. The ATR pathway: fine-tuning the fork. DNA Repair (Amst.) 6, 953–966 (2007).
Andersen, P.L., Xu, F. & Xiao, W. Eukaryotic DNA damage tolerance and translesion synthesis through covalent modifications of PCNA. Cell Res. 18, 162–173 (2008).
Moldovan, G.L., Pfander, B. & Jentsch, S. PCNA, the maestro of the replication fork. Cell 129, 665–679 (2007).
McCulloch, S.D. & Kunkel, T.A. The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 18, 148–161 (2008).
Yang, W. & Woodgate, R. What a difference a decade makes: insights into translesion DNA synthesis. Proc. Natl. Acad. Sci. USA 104, 15591–15598 (2007).
Prakash, S., Johnson, R.E. & Prakash, L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74, 317–353 (2005).
Friedberg, E.C., Lehmann, A.R. & Fuchs, R.P. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol. Cell 18, 499–505 (2005).
Fujii, S. & Fuchs, R.P. Interplay among replicative and specialized DNA polymerases determines failure or success of translesion synthesis pathways. J. Mol. Biol. 372, 883–893 (2007).
Fujii, S. & Fuchs, R.P. Defining the position of the switches between replicative and bypass DNA polymerases. EMBO J. 23, 4342–4352 (2004).
Lawrence, C.W. & Christensen, R. UV mutagenesis in radiation-sensitive strains of yeast. Genetics 82, 207–232 (1976).
Lemontt, J.F. Mutants of yeast defective in mutation induced by ultraviolet light. Genetics 68, 21–33 (1971).
Johnson, R.E., Prakash, S. & Prakash, L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science 283, 1001–1004 (1999).
McCulloch, S.D. et al. Preferential cis-syn thymine dimer bypass by DNA polymerase eta occurs with biased fidelity. Nature 428, 97–100 (2004).
Zhang, Y. et al. Error-free and error-prone lesion bypass by human DNA polymerase kappa in vitro. Nucleic Acids Res. 28, 4138–4146 (2000).
Ogi, T., Shinkai, Y., Tanaka, K. & Ohmori, H. Polkappa protects mammalian cells against the lethal and mutagenic effects of benzo[a]pyrene. Proc. Natl. Acad. Sci. USA 99, 15548–15553 (2002).
Masutani, C. et al. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 399, 700–704 (1999).
Broomfield, S., Chow, B.L. & Xiao, W. MMS2, encoding a ubiquitin-conjugating-enzyme-like protein, is a member of the yeast error-free postreplication repair pathway. Proc. Natl. Acad. Sci. USA 95, 5678–5683 (1998).
Broomfield, S., Hryciw, T. & Xiao, W. DNA postreplication repair and mutagenesis in Saccharomyces cerevisiae. Mutat. Res. 486, 167–184 (2001).
Chiu, R.K. et al. Lysine 63-polyubiquitination guards against translesion synthesis-induced mutations. PLoS Genet. 2, e116 (2006).
Xiao, W., Chow, B.L., Broomfield, S. & Hanna, M. The Saccharomyces cerevisiae RAD6 group is composed of an error-prone and two error-free postreplication repair pathways. Genetics 155, 1633–1641 (2000).
Higgins, N.P., Kato, K. & Strauss, B. A model for replication repair in mammalian cells. J. Mol. Biol. 101, 417–425 (1976).
Lehmann, A.R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J. Mol. Biol. 66, 319–337 (1972).
Zhang, H. & Lawrence, C.W. The error-free component of the RAD6/RAD18 DNA damage tolerance pathway of budding yeast employs sister-strand recombination. Proc. Natl. Acad. Sci. USA 102, 15954–15959 (2005).
Fujiwara, Y. & Tatsumi, M. Replicative bypass repair of ultraviolet damage to DNA of mammalian cells: caffeine sensitive and caffeine resistant mechanisms. Mutat. Res. 37, 91–110 (1976).
Cotta-Ramusino, C. et al. Exo1 processes stalled replication forks and counteracts fork reversal in checkpoint-defective cells. Mol. Cell 17, 153–159 (2005).
Lopes, M., Foiani, M. & Sogo, J.M. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell 21, 15–27 (2006).
Papouli, E. et al. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol. Cell 19, 123–133 (2005).
Pfander, B., Moldovan, G.L., Sacher, M., Hoege, C. & Jentsch, S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature 436, 428–433 (2005).
Frampton, J. et al. Postreplication repair and PCNA modification in Schizosaccharomyces pombe. Mol. Biol. Cell 17, 2976–2985 (2006).
Stelter, P. & Ulrich, H.D. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425, 188–191 (2003).
Hoege, C., Pfander, B., Moldovan, G.L., Pyrowolakis, G. & Jentsch, S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419, 135–141 (2002).
Gohler, T., Munoz, I.M., Rouse, J. & Blow, J.J. PTIP/Swift is required for efficient PCNA ubiquitination in response to DNA damage. DNA Repair (Amst.) 7, 775–787 (2008).
Chang, D.J., Lupardus, P.J. & Cimprich, K.A. Monoubiquitination of proliferating cell nuclear antigen induced by stalled replication requires uncoupling of DNA polymerase and mini-chromosome maintenance helicase activities. J. Biol. Chem. 281, 32081–32088 (2006).
Leach, C.A. & Michael, W.M. Ubiquitin/SUMO modification of PCNA promotes replication fork progression in Xenopus laevis egg extracts. J. Cell Biol. 171, 947–954 (2005).
Watanabe, K. et al. Rad18 guides poleta to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 23, 3886–3896 (2004).
Kannouche, P.L., Wing, J. & Lehmann, A.R. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell 14, 491–500 (2004).
Unk, I. et al. Human HLTF functions as a ubiquitin ligase for proliferating cell nuclear antigen polyubiquitination. Proc. Natl. Acad. Sci. USA 105, 3768–3773 (2008).
Unk, I. et al. Human SHPRH is a ubiquitin ligase for Mms2-Ubc13-dependent polyubiquitylation of proliferating cell nuclear antigen. Proc. Natl. Acad. Sci. USA 103, 18107–18112 (2006).
Motegi, A. et al. Human SHPRH suppresses genomic instability through proliferating cell nuclear antigen polyubiquitination. J. Cell Biol. 175, 703–708 (2006).
Motegi, A. et al. Polyubiquitination of proliferating cell nuclear antigen by HLTF and SHPRH prevents genomic instability from stalled replication forks. Proc. Natl. Acad. Sci. USA 105, 12411–12416 (2008).
Bi, X. et al. Rad18 regulates DNA polymerase kappa and is required for recovery from S-phase checkpoint-mediated arrest. Mol. Cell. Biol. 26, 3527–3540 (2006).
Bienko, M. et al. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310, 1821–1824 (2005).
Guo, C., Tang, T.S., Bienko, M., Dikic, I. & Friedberg, E.C. Requirements for the interaction of mouse Polkappa with ubiquitin and its biological significance. J. Biol. Chem. 283, 4658–4664 (2008).
Plosky, B.S. et al. Controlling the subcellular localization of DNA polymerases iota and eta via interactions with ubiquitin. EMBO J. 25, 2847–2855 (2006).
Garg, P. & Burgers, P.M. Ubiquitinated proliferating cell nuclear antigen activates translesion DNA polymerases eta and REV1. Proc. Natl. Acad. Sci. USA 102, 18361–18366 (2005).
Haracska, L., Unk, I., Prakash, L. & Prakash, S. Ubiquitylation of yeast proliferating cell nuclear antigen and its implications for translesion DNA synthesis. Proc. Natl. Acad. Sci. USA 103, 6477–6482 (2006).
Sabbioneda, S. et al. Effect of proliferating cell nuclear antigen ubiquitination and chromatin structure on the dynamic properties of the Y-family DNA polymerases. Mol. Biol. Cell 19, 5193–5202 (2008).
Niimi, A. et al. Regulation of proliferating cell nuclear antigen ubiquitination in mammalian cells. Proc. Natl. Acad. Sci. USA 105, 16125–16130 (2008).
Waters, L.S. & Walker, G.C. The critical mutagenic translesion DNA polymerase Rev1 is highly expressed during G(2)/M phase rather than S phase. Proc. Natl. Acad. Sci. USA 103, 8971–8976 (2006).
Masuda, Y. & Kamiya, K. Role of single-stranded DNA in targeting REV1 to primer termini. J. Biol. Chem. 281, 24314–24321 (2006).
Tissier, A. et al. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase pol eta and REVl protein. DNA Repair (Amst.) 3, 1503–1514 (2004).
Hochstrasser, M. Lingering mysteries of ubiquitin-chain assembly. Cell 124, 27–34 (2006).
Ulrich, H.D. & Jentsch, S. Two RING finger proteins mediate cooperation between ubiquitin-conjugating enzymes in DNA repair. EMBO J. 19, 3388–3397 (2000).
Huang, T.T. et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat. Cell Biol. 8, 339–347 (2006).
Gangavarapu, V. et al. Mms2-Ubc13-dependent and -independent roles of Rad5 ubiquitin ligase in postreplication repair and translesion DNA synthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 26, 7783–7790 (2006).
Pages, V. et al. Requirement of Rad5 for DNA polymerase zeta-dependent translesion synthesis in Saccharomyces cerevisiae. Genetics 180, 73–82 (2008).
Chen, S., Davies, A.A., Sagan, D. & Ulrich, H.D. The RING finger ATPase Rad5p of Saccharomyces cerevisiae contributes to DNA double-strand break repair in a ubiquitin-independent manner. Nucleic Acids Res. 33, 5878–5886 (2005).
Flaus, A., Martin, D.M., Barton, G.J. & Owen-Hughes, T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 34, 2887–2905 (2006).
Blastyak, A. et al. Yeast Rad5 protein required for postreplication repair has a DNA helicase activity specific for replication fork regression. Mol. Cell 28, 167–175 (2007).
Constantinou, A. et al. Werner's syndrome protein (WRN) migrates Holliday junctions and co-localizes with RPA upon replication arrest. EMBO Rep. 1, 80–84 (2000).
Karow, J.K., Constantinou, A., Li, J.L., West, S.C. & Hickson, I.D. The Bloom's syndrome gene product promotes branch migration of holliday junctions. Proc. Natl. Acad. Sci. USA 97, 6504–6508 (2000).
Machwe, A., Xiao, L., Groden, J. & Orren, D.K. The Werner and Bloom syndrome proteins catalyze regression of a model replication fork. Biochemistry 45, 13939–13946 (2006).
Ralf, C., Hickson, I.D. & Wu, L. The Bloom's syndrome helicase can promote the regression of a model replication fork. J. Biol. Chem. 281, 22839–22846 (2006).
Gari, K., Decaillet, C., Stasiak, A.Z., Stasiak, A. & Constantinou, A. The Fanconi anemia protein FANCM can promote branch migration of Holliday junctions and replication forks. Mol. Cell 29, 141–148 (2008).
Gari, K., Decaillet, C., Delannoy, M., Wu, L. & Constantinou, A. Remodeling of DNA replication structures by the branch point translocase FANCM. Proc. Natl. Acad. Sci. USA 105, 16107–16112 (2008).
Sun, W. et al. The FANCM ortholog Fml1 promotes recombination at stalled replication forks and limits crossing over during DNA double-strand break repair. Mol. Cell 32, 118–128 (2008).
Kanagaraj, R., Saydam, N., Garcia, P.L., Zheng, L. & Janscak, P. Human RECQ5beta helicase promotes strand exchange on synthetic DNA structures resembling a stalled replication fork. Nucleic Acids Res. 34, 5217–5231 (2006).
Bienz, M. The PHD finger, a nuclear protein-interaction domain. Trends Biochem. Sci. 31, 35–40 (2006).
Peng, J. & Wysocka, J. It takes a PHD to SUMO. Trends Biochem. Sci. 33, 191–194 (2008).
Iyer, L.M., Babu, M.M. & Aravind, L. The HIRAN domain and recruitment of chromatin remodeling and repair activities to damaged DNA. Cell Cycle 5, 775–782 (2006).
Davies, A.A., Huttner, D., Daigaku, Y., Chen, S. & Ulrich, H.D. Activation of ubiquitin-dependent DNA damage bypass is mediated by replication protein a. Mol. Cell 29, 625–636 (2008).
Pacek, M. & Walter, J.C. A requirement for MCM7 and Cdc45 in chromosome unwinding during eukaryotic DNA replication. EMBO J. 23, 3667–3676 (2004).
Byun, T.S., Pacek, M., Yee, M.C., Walter, J.C. & Cimprich, K.A. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 19, 1040–1052 (2005).
Bailly, V., Lauder, S., Prakash, S. & Prakash, L. Yeast DNA repair proteins Rad6 and Rad18 form a heterodimer that has ubiquitin conjugating, DNA binding, and ATP hydrolytic activities. J. Biol. Chem. 272, 23360–23365 (1997).
Tsuji, Y. et al. Recognition of forked and single-stranded DNA structures by human RAD18 complexed with RAD6B protein triggers its recruitment to stalled replication forks. Genes Cells 13, 343–354 (2008).
Zou, L. Single- and double-stranded DNA: building a trigger of ATR-mediated DNA damage response. Genes Dev. 21, 879–885 (2007).
MacDougall, C.A., Byun, T.S., Van, C., Yee, M.C. & Cimprich, K.A. The structural determinants of checkpoint activation. Genes Dev. 21, 898–903 (2007).
Yang, X.H., Shiotani, B., Classon, M. & Zou, L. Chk1 and Claspin potentiate PCNA ubiquitination. Genes Dev. 22, 1147–1152 (2008).
Sabbioneda, S. et al. The 9–1-1 checkpoint clamp physically interacts with polzeta and is partially required for spontaneous polzeta-dependent mutagenesis in Saccharomyces cerevisiae. J. Biol. Chem. 280, 38657–38665 (2005).
Hirano, Y. & Sugimoto, K. ATR homolog Mec1 controls association of DNA polymerase zeta-Rev1 complex with regions near a double-strand break. Curr. Biol. 16, 586–590 (2006).
Kai, M. & Wang, T.S. Checkpoint activation regulates mutagenic translesion synthesis. Genes Dev. 17, 64–76 (2003).
Matsuoka, S. et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160–1166 (2007).
Welchman, R.L., Gordon, C. & Mayer, R.J. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell Biol. 6, 599–609 (2005).
Kim, H.T. et al. Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages. J. Biol. Chem. 282, 17375–17386 (2007).
Acknowledgements
The authors thank T. Wandless, A. Hahn, J.-R. Lin and M. Zeman for helpful discussions and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, D., Cimprich, K. DNA damage tolerance: when it's OK to make mistakes. Nat Chem Biol 5, 82–90 (2009). https://doi.org/10.1038/nchembio.139
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.139