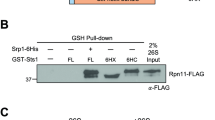
Abstract
The ubiquitin-like protein SUMO-1 (small ubiquitin-related modifier 1) is covalently attached to substrate proteins by ligases and cleaved by isopeptidases. Yeast has two SUMO-1-deconjugating enzymes, Ulp1 and Ulp2, which are located at nuclear pores and in the nucleoplasm, respectively. Here we show that the catalytic C-domain of Ulp1 must be excluded from the nucleoplasm for cell viability. This is achieved by the noncatalytic N-domain, which tethers Ulp1 to the nuclear pores. The bulk of cellular Ulp1 is not associated with nucleoporins but instead associates with three karyopherins (Pse1, Kap95 and Kap60), in a complex that is not dissociated by RanGTP in vitro. The Ulp1 N-domain has two distinct binding sites for Pse1 and Kap95/Kap60, both of which are required for anchoring to the nuclear pore complex. We propose that Ulp1 is tethered to the nuclear pores by a Ran-insensitive interaction with karyopherins associated with nucleoporins. This location could allow Ulp1 to remove SUMO-1 from sumoylated cargo proteins during their passage through the nuclear pore channel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Laney, J. D. & Hochstrasser, M. Substrate targeting in the ubiquitin system. Cell 97, 427–430 (1999).
Johnson, E. S., Schwienhorst, I., Dohmen, R. J. & Blobel, G. The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 16, 5509–5519 (1997).
Johnson, E. S. & Blobel, G. Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. J. Biol. Chem. 272, 26799–26802 (1997).
Hochstrasser, M. All in the ubiquitin family. Science 289, 563–564 (2000).
Melchior, F. SUMO-1 — nonclassical ubiquitin. Annu. Rev. Cell. Dev. Biol. 16, 591–626 (2000).
Johnson, E. S. & Blobel, G. Cell cycle-regulated attachment of the ubiquitin-related protein SUMO to the yeast septins. J. Cell Biol. 147, 981–994 (1999).
Mahajan, R., Delphin, C., Guan, T., Gerace, L. & Melchior, F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88, 97–107 (1997).
Saitoh, H., Pu, R., Cavenagh, M. & Dasso, M. RanBP2 associates with Ubc9p and a modified form of RanGAP1. Proc. Natl Acad. Sci. USA 94, 3736–3741 (1997).
Pichler, A., Gast, A., Seeler, J. S., Dejean, A. & Melchior, F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 108, 109–120 (2002).
Li, S. J. & Hochstrasser, M. A new protease required for cell-cycle progression in yeast. Nature 398, 246–251 (1999).
Li, S. J. & Hochstrasser, M. The yeast ULP2 (SMT4) gene encodes a novel protease specific for the ubiquitin-like Smt3 protein. Mol. Cell. Biol. 20, 2367–2377 (2000).
Mossessova, E. & Lima, C. D. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol. Cell 5, 865–876 (2000).
Gadal, O. et al. Nuclear export of 60S ribosomal subunits depends on Xpo1p and requires a nuclear export sequence-containing factor, Nmd3p, that associates with the large subunit protein Rpl10p. Mol. Cell. Biol. 21, 3405–3415 (2001).
Galani, K., Grosshans, H., Deinert, K., Hurt, E. C. & Simos, G. The intracellular location of two aminoacyl-tRNA synthetases depends on complex formation with Arc1p. EMBO J. 23, 6889–6898 (2001).
Rout, M. P. et al. The yeast nuclear pore complex: composition, architecture and transport mechanism. J. Cell Biol. 148, 635–651 (2000).
Takahashi, Y., Mizoi, J., Toh-E, A. & Kikuchi, Y. Yeast Ulp1, an Smt3-specific protease, associates with nucleoporins. J. Biochem. (Tokyo) 128, 723–725 (2000).
Strahm, Y. et al. The RNA export factor Gle1p is located on the cytoplasmic fibrils of the NPC and physically interacts with the FG-nucleoporin Rip1p, the DEAD-box protein Rat8p/Dbp5p and a new protein Ymr255p. EMBO J. 18, 5761–5777 (1999).
Simos, G. et al. The yeast protein Arc1p binds to tRNA and functions as a cofactor for the methionyl- and glutamyl-tRNA synthetases. EMBO J. 15, 5437–5448 (1996).
Görlich, D. & Kutay, U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell. Dev. Biol. 15, 607–660 (1999).
Künzler, M., Gerstberger, T., Stutz, F., Bischoff, F. R. & Hurt. E. Yeast Ran-binding protein 1 (Yrb1) shuttles between the nucleus and cytoplasm and is exported from the nucleus via a CRM1 (XPO1)-dependent pathway. Mol. Cell. Biol. 20, 4295–4308 (2000).
Schlenstedt, G. et al. Yrb4p, a yeast Ran-GTP-binding protein involved in import of ribosomal protein L25 into the nucleus. EMBO J. 16, 6237–6249 (1997).
Ho, J. H., Kallstrom, G. & Johnson, A. W. Nmd3p is a Crm1p-dependent adapter protein for nuclear export of the large ribosomal subunit. J. Cell Biol. 151, 1057–1066 (2000).
Neville, M. & Rosbash, M. The NES-Crm1p export pathway is not a major mRNA export route in Saccharomyces cerevisiae. EMBO J. 18, 3746–3756 (1999).
Taylor, D. L., Ho, J. C., Oliver, A. & Watts, F. Z. Cell-cycle-dependent localisation of Ulp1, a Schizosaccharomyces pombe Pmt3 (SUMO)-specific protease. J. Cell Sci. 115, 1113–1122 (2002).
Hang, J. & Dasso, M. Association of the human SUMO-1 protease SENP2 with the nuclear pore. J. Biol. Chem. 277, 19961–19966 (2002).
Zhang, H., Saitoh, H. & Matunis, M. J. Enzymes of the SUMO modification pathway localize to filaments of the nuclear pore complex. Mol. Cell. Biol. 22, 6498–6508 (2002).
Shah, S., Tugendreich, S. & Forbes, D. Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr. J. Cell Biol. 141, 31–49 (1998).
Kirsh, O. et al. The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. EMBO J. 21, 2682–2691 (2002).
Bassler, J. et al. Identification of a 60S preribosomal particle that is closely linked to nuclear export. Mol. Cell 8, 517–529 (2001).
Hellmuth, K. et al. Yeast Los1p has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol. Cell. Biol. 18, 6374–6386 (1998).
Siniossoglou, S. et al. Structure and assembly of the Nup84p complex. J. Cell Biol. 149, 41–54 (2000).
Seedorf, M. & Silver, P. A. Importin/karyopherin protein family members required for mRNA export from the nucleus. Proc. Natl Acad. Sci. USA 94, 8590–8595 (1997).
Acknowledgements
E.H. was supported by grants from the Deutsche Forschungsgemeinschaft (Leibniz-Programm) and Fonds der Chemischen Industrie. V.G.P. is the recipient of a long-term fellowship from the Human Frontier Science Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Tables
Table 1: Yeast strains (DOC 28 kb)
Table 2: Plasmids and constructions
Legend to supplementary Figure 1.
Supplementary Figure
Fig. 1. Levels of Ulp1-GFP are not altered upon ovexpression of Yrb4ΔN or in the pse1-1 strain when shifted to restrictive temperature. (JPG 391 kb)
Rights and permissions
About this article
Cite this article
Panse, V., Küster, B., Gerstberger, T. et al. Unconventional tethering of Ulp1 to the transport channel of the nuclear pore complex by karyopherins. Nat Cell Biol 5, 21–27 (2003). https://doi.org/10.1038/ncb893
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb893