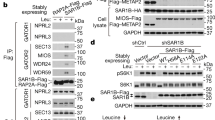
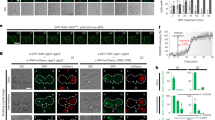
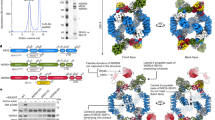
Abstract
Target of Rapamycin (TOR) mediates a signalling pathway that couples amino acid availability to S6 kinase (S6K) activation, translational initiation and cell growth1,2. Here, we show that tuberous sclerosis 1 (Tsc1) and Tsc2, tumour suppressors that are responsible for the tuberous sclerosis syndrome3,4, antagonize this amino acid–TOR signalling pathway. We show that Tsc1 and Tsc2 can physically associate with TOR and function upstream of TOR genetically. In Drosophila melanogaster and mammalian cells, loss of Tsc1 and Tsc2 results in a TOR-dependent increase of S6K activity. Furthermore, although S6K is normally inactivated in animal cells in response to amino acid starvation, loss of Tsc1–Tsc2 renders cells resistant to amino acid starvation. We propose that the Tsc1–Tsc2 complex antagonizes the TOR-mediated response to amino acid availability. Our studies identify Tsc1 and Tsc2 as regulators of the amino acid–TOR pathway and provide a new paradigm for how proteins involved in nutrient sensing function as tumour suppressors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thomas, G. & Hall, M. N. Curr. Opin. Cell Biol. 9, 782–787 (1997).
Gingras, A. C., Raught, B. & Sonenberg, N. Genes Dev. 15, 807–826 (2001).
van Slegtenhorst, M. et al. Science 277, 805–808 (1997).
The European Chromosome 16 Tuberous Sclerosis Consortium. Cell 75, 1305–1315 (1993).
Kuruvilla, F. G. & Schreiber, S. L. Chem. Biol. 6, R129–R136 (1999).
Hara, K. et al. J. Biol. Chem. 273, 14484–14494 (1998).
Stocker, H. & Hafen, E. Curr. Opin. Genet. Dev. 10, 529–535 (2000).
Tapon, N., Ito, N., Dickson, B. J., Treisman, J. E. & Hariharan, I. K. Cell 105, 345–355 (2001).
Potter, C. J., Huang, H. & Xu, T. Cell 105, 357–368 (2001).
Gao, X. & Pan, D. Genes Dev. 15, 1383–1392 (2001).
Young, J. & Povey, S. Mol. Med. Today 4, 313–319 (1998).
Nellist, M. et al. J. Biol. Chem. 274, 35647–35652 (1999).
van Slegtenhorst, M. et al. Hum. Mol. Genet. 7, 1053–1057 (1998).
Clemens, J. C. et al. Proc. Natl Acad. Sci. USA 97, 6499–6503 (2000).
Weng, Q. P. et al. J. Biol. Chem. 273, 16621–16629 (1998).
Radimerski, T. et al. Nature Cell Biol. 4, 251–255 (2002).
Kwiatkowski, D. J. et al. Hum. Mol. Genet. 11, 525–534 (2002).
Miron, M. et al. Nature Cell Biol. 3, 596–601 (2001).
Yeung, R. S. et al. Proc. Natl Acad. Sci. USA 91, 11413–11416 (1994).
Xiao, G.-H., Shoarinejad, F., Jin, F., Golemis, E. A. & Yeung, R. S. J. Biol. Chem. 272, 6097–6100 (1997).
Kobayashi, T. et al. Proc. Natl Acad. Sci. USA 98, 8762–8767 (2001).
Hanahan, D. & Weinberg, R. A. Cell 100, 57–70 (2000).
Ito, N. & Rubin, G. M. Cell 96, 529–539 (1999).
Montagne, J. et al. Science 285, 2126–2129 (1999).
Oldham, S., Montagne, J., Radimerski, T., Thomas, G. & Hafen, E. Genes Dev. 14, 2689–2694 (2000).
Zhang, H., Stallock, J. P., Ng, J. C., Reinhard, C. & Neufeld, T. P. Genes Dev. 14, 2712–2724 (2000).
Chen, E. H. & Olson, E. N. Dev. Cell 1, 705–715 (2001).
Seegmiller, A. C. et al. Dev. Cell 2, 229–238 (2002).
Echalier, G. Drosophila Cells in Culture (Academic Press, New York, 1997).
Dennis, P. B. et al. Science 294, 1102–1105 (2001).
Benvenuto, G. et al. Oncogene 19, 6306–6316 (2000).
Plank, T. L., Yeung, R. S. & Henske, E. P. Cancer Res. 58, 4766–4770 (1998).
Acknowledgements
We would like to thank M. Brown, E. Chen, J. Chen, J. Stull and K. Wharton for critical reading of the manuscript. We thank E. Hafen and T. Neufeld for fly stocks, N. Ito and M. Stewart for antibodies, P. Jones for amino acid measurement and the University of Texas Southwestern Molecular and Cellular Imaging Facility for assistance with eye sections. D.J.P. is Virginia Murchison Linthicum Endowed Scholar in Medical Science, and is supported by the National Institutes of Health (GM62323) and American Heart Association (0130222N).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gao, X., Zhang, Y., Arrazola, P. et al. Tsc tumour suppressor proteins antagonize amino-acid–TOR signalling. Nat Cell Biol 4, 699–704 (2002). https://doi.org/10.1038/ncb847
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb847