Abstract
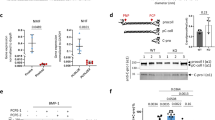
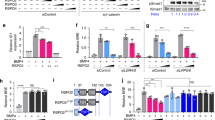
Connective-tissue growth factor (CTGF) is a secreted protein implicated in multiple cellular events including angiogenesis, skeletogenesis and wound healing1. It is a member of the CCN family of secreted proteins, named after CTGF, cysteine-rich 61 (CYR61), and nephroblastoma overexpressed (NOV) proteins. The molecular mechanism by which CTGF or other CCN proteins regulate cell signalling is not known. CTGF contains a cysteine-rich domain (CR) similar to those found in chordin and other secreted proteins2, which in some cases have been reported to function as bone morphogenetic protein (BMP) and TGF-β binding domains3,4,5,6. Here we show that CTGF directly binds BMP4 and TGF-β1 through its CR domain. CTGF can antagonize BMP4 activity by preventing its binding to BMP receptors and has the opposite effect, enhancement of receptor binding, on TGF-β1. These results show that CTGF inhibits BMP and activates TGF-β signals by direct binding in the extracellular space.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Moussad, E. E. & Brigstock, D. R. Mol. Genet. Metab. 71, 276–292 (2000).
Abreu, J., Coffinier, C., Larraín, J., Oelgeschläger, M. & De Robertis, E. M. Gene 287, 39–47 (2002).
Zhu, Y., Oganesian, A., Keene, D.R. & Sandell, L. J. J. Cell Biol. 144, 1069–1080 (1999).
Larraín, J. et al. Development 127, 821–830 (2000).
Nakayama, N. et al. Dev. Biol. 232, 372–387 (2001).
Sakuta, H. et al. Science 293, 111–115 (2001).
Hunt, L.T. & Barker, W.C. Biochem. Biophys. Res. Commun. 144, 876–882 (1987).
Bork, P. FEBS Lett. 327, 125–130 (1993).
Smith, W.C. & Harland, R.M. Cell 70, 829–840 (1992).
Sasai, Y. et al. Cell 79, 779–790 (1994).
Masuhara, K. et al. Bone 16, 91–96. (1995).
Iemura, S. et al. Proc. Natl. Acad. Sci. USA 95, 9337–9342 (1998).
Piccolo, S., Sasai, Y., Lu, B. & De Robertis, E. M. Cell 86, 589–598 (1996).
Katagiri, T. et al. Biochem. Biophys. Res. Commun. 172, 295–299 (1990).
Persson, V. et al. FEBS Lett. 434, 83–87 (1998).
Larraín, J. et al. Development 128, 4439–44347 (2001).
Massagué, J. Methods Enzymol. 46, 174–195 (1987).
Wrana, J. L. et al. Cell 71, 1003–1014 (1992).
Robson, P., Stein, P., Zhou, B., Schultz, R. M. & Baldoin, H. S. Dev. Biol. 234, 317–329 (2001).
Segarini, P. R. et al. J. Biol. Chem. 276, 40659–40667 (2001).
Kireeva, M. L. et al. Exp. Cell Res. 233, 63–77 (1997).
Roberts, A. B. et al. Proc. Natl. Acad. Sci. USA 83, 4167–4171 (1986).
Frazier, K., Williams, S., Kothapalli, D., Klapper, H. & Grotendorst, G. R. J. Invest. Dermatol. 107, 404–411 (1996).
Grotendorst, G. R., Okochi, H. & Hayashi, N. Cell Growth Differ. 7, 469–480 (1996).
Holmes, A. et al. J. Biol. Chem. 276, 10594–10601 (2001).
Kothapalli, D., Frazier, K. S., Welply, A., Segarini, P. R. & Grotendorst, G. R. Cell Growth Differ. 8, 61–68 (1997).
Kim, H. S. et al. Proc. Natl Acad. Sci. USA 94, 12981–12986 (1997).
Adams, J. C. & Tucker, R. P. Dev. Dyn. 218, 280–299 (2000).
Inoki, I. et al. FASEB J. 16, 219–221 (2002).
Pearce, J. J., Penny, G. & Rossant, J. Dev. Biol. 209, 98–110 (1999).
Acknowledgements
We thank K. M. Lyons and S. Ivkovic for unpublished information, K. Masuhara and C.H. Heldin for antibodies, J. Massague for reporter plasmid, Naoto Ueno for the protocol to immobilize BMP4 to Biacore chips, M. L. King and Z. Ying for entering the CTGF full-length sequence in GenBank, S.-Y. Li and A. Cuellar for technical assistance, and C. Coffinier, E. Delot, J. I. Kim, J. Larraín, K. M. Lyons, M. Oelgeschläger, E. Pera, O. Wessely for discussions and comments on the manuscript. J.G.A. was a Latin American PEW fellow. This work was supported by the National Institutes of Health (R37 HD21502-16). E.M.D.R. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary figures
Figure S1. Expression pattern of CTGF in Xenopus embryogenesis detected by in situ hybridization. dorsal aspect of the somite (f-i). (PDF 485 kb)
Figure S2. CTGF constructs and expression of CTGF proteins in human 293T cells, Xenopus embryos and Drosophila S2 cells.
Figure S3. Synergy between TGF-β1 and CTGF: Induction of striking morphological changes in Mv1Lu cells.
Rights and permissions
About this article
Cite this article
Abreu, J., Ketpura, N., Reversade, B. et al. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol 4, 599–604 (2002). https://doi.org/10.1038/ncb826
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb826
This article is cited by
-
Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Development of cochlear spiral ligament fibrocytes of the common marmoset, a nonhuman model animal
Scientific Reports (2023)
-
Rescue of murine hind limb ischemia via angiogenesis and lymphangiogenesis promoted by cellular communication network factor 2
Scientific Reports (2023)
-
Role of PDGFRA+ cells and a CD55+ PDGFRALo fraction in the gastric mesenchymal niche
Nature Communications (2023)
-
CCN2/CTGF promotes liver fibrosis through crosstalk with the Slit2/Robo signaling
Journal of Cell Communication and Signaling (2023)