Abstract
Development of the nervous system requires extensive axonal and dendritic growth during which neurons massively increase their surface area. Here we report that the endoplasmic reticulum (ER)-resident SNARE Sec22b has a conserved non-fusogenic function in plasma membrane expansion. Sec22b is closely apposed to the plasma membrane SNARE syntaxin1. Sec22b forms a trans-SNARE complex with syntaxin1 that does not include SNAP23/25/29, and does not mediate fusion. Insertion of a long rigid linker between the SNARE and transmembrane domains of Sec22b extends the distance between the ER and plasma membrane, and impairs neurite growth but not the secretion of VSV-G. In yeast, Sec22 interacts with lipid transfer proteins, and inhibition of Sec22 leads to defects in lipid metabolism at contact sites between the ER and plasma membrane. These results suggest that close apposition of the ER and plasma membrane mediated by Sec22 and plasma membrane syntaxins generates a non-fusogenic SNARE bridge contributing to plasma membrane expansion, probably through non-vesicular lipid transfer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
11 July 2014
In the version of this Article originally published, the first sentence of the Immunoprecipitation section in Methods was incomplete. The correct wording reads: "Whole mouse brain or transfected COS7 cells were homogenized in homogenization buffer (50 mM Hepes pH 7.2, 100 mM NaCl, 4 mM EGTA, 2 mM MgCl2 and protein inhibitors (Roche, Complete without EDTA)) at 4 °C. Proteins were then extracted by addition of 1% final concentration of Triton X-100 and lysis at 4 °C for 2 h or 30 min respectively. The insoluble material was removed by centrifugation at 13,000 r.p.m. for 30 min with a Beckman tabletop centrifuge. Total protein in the extracts was quantified with Bradford assays." This has been corrected in the online versions of the Article.
References
Hanus, C. & Ehlers, M. D. Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic 9, 1437–1445 (2008)10.1111/j.1600-0854.2008.00775.x
Pfenninger, K. H. Plasma membrane expansion: a neuron’s Herculean task. Nat. Rev. Neurosci. 10, 251–261 (2009)10.1038/nrn2593
Futerman, A. H. & Banker, G. A. The economics of neurite outgrowth–the addition of new membrane to growing axons. Trends Neurosci. 19, 144–149 (1996).
Martinez-Arca, S., Alberts, P., Zahraoui, A., Louvard, D. & Galli, T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J. Cell Biol. 149, 889–900 (2000).
Alberts, P. et al. Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion. Mol. Biol. Cell 14, 4207–4220 (2003)10.1091/mbc.E03-03-0147
Gupton, S. L. & Gertler, F. B. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev. Cell 18, 725–736 (2010)10.1016/j.devcel.2010.02.017
Schulte, C., Racchetti, G., D’Alessandro, R. & Meldolesi, J. A new form of neurite outgrowth sustained by the exocytosis of enlargeosomes expressed under the control of REST. Traffic 11, 1304–1314 (2010)10.1111/j.1600-0854.2010.01095.x
Stefan, C. J. et al. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell 144, 389–401 (2011)10.1016/j.cell.2010.12.034
Tavassoli, S. et al. Plasma membrane–endoplasmic reticulum contact sites regulate phosphatidylcholine synthesis. EMBO Rep. 14, 434–440 (2013)10.1038/embor.2013.36
Voelker, D. R. Genetic and biochemical analysis of non-vesicular lipid traffic. Annu. Rev. Biochem. 78, 827–856 (2009)10.1146/annurev.biochem.78.081307.112144
Jahn, R. & Scheller, R. H. SNAREs–engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7, 631–643 (2006)10.1038/nrm2002
Gao, Y. et al. Single reconstituted neuronal SNARE complexes zipper in three distinct stages. Science 337, 1340–1343 (2012)10.1126/science.1224492
Hernandez, J. M. et al. Membrane fusion intermediates via directional and full assembly of the SNARE complex. Science 336, 1581–1584 (2012)10.1126/science.1221976
Martinez-Arca, S. et al. A common exocytotic mechanism mediates axonal and dendritic outgrowth. J. Neurosci. 21, 3830–3838 (2001).
Danglot, L. et al. Role of TI-VAMP and CD82 in EGFR cell-surface dynamics and signaling. J. Cell Sci. 123, 723–735 (2010)10.1242/jcs.062497
Schoch, S. et al. SNARE function analysed in synaptobrevin/VAMP knockout mice. Science 294, 1117–1122 (2001)10.1126/science.1064335
Zylbersztejn, K. et al. The vesicular SNARE synaptobrevin is required for semaphorin 3A axonal repulsion. J. Cell Biol. 196, 37–46 (2012)10.1083/jcb.201106113
Pfenninger, K. H. Transport and insertion of membrane components into processes of growing neurons. Neurosci. Res. Prog. Bull. 20, 73–79 (1981).
Lockerbie, R. O., Miller, V. E. & Pfenninger, K. H. Regulated plasmalemmal expansion in nerve growth cones. J. Cell Biol. 112, 1215–1227 (1991).
Liu, Y. T., Flanagan, J. J. & Barlowe, C. Sec22p export from the endoplasmic reticulum is independent of SNARE pairing. J. Biol. Chem. 279, 27225–27232 (2004).
Burgo, A. et al. A molecular network for the transport of the TI-VAMP/VAMP7 vesicles from cell center to periphery. Dev. Cell 23, 166–180 (2012)10.1016/j.devcel.2012.04.019
Burgo, A. et al. Role of Varp, a Rab21 exchange factor and TI-VAMP/VAMP7 partner, in neurite growth. EMBO Rep. 10, 1117–1124 (2009)10.1038/embor.2009.186
Rossi, V. et al. Longins and their longin domains: regulated SNAREs and multifunctional SNARE regulators. Trends Biochem. Sci. 29, 682–688 (2004)10.1016/j.tibs.2004.10.002
Chaineau, M., Danglot, L. & Galli, T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett. 583, 3817–3826 (2009)10.1016/j.febslet.2009.10.026
Mancias, J. D. & Goldberg, J. The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Mol. Cell 26, 403–414 (2007)10.1016/j.molcel.2007.03.017
Cebrian, I. et al. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell 147, 1355–1368 (2011)10.1016/j.cell.2011.11.021
Xu, D., Joglekar, A. P., Williams, A. L. & Hay, J. C. Subunit structure of a mammalian ER/Golgi SNARE complex. J. Biol. Chem. 275, 39631–39639 (2000)10.1074/jbc.M007684200
Martinez-Arca, S. et al. A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc. Natl Acad. Sci. USA 100, 9011–9016 (2003)10.1073/pnas.1431910100
Becher, A., Drenckhahn, A., Pahner, I. & AhnertHilger, G. The synaptophysin-synaptobrevin complex is developmentally upregulated in cultivated neurons but is absent in neuroendocrine cells. Eur. J. Cell Biol. 78, 650–656 (1999).
Lang, T. et al. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 20, 2202–2213 (2001).
Touret, N. et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell 123, 157–170 (2005)10.1016/j.cell.2005.08.018
Izeddin, I. et al. Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS ONE 6, e15611 (2011)10.1371/journal.pone.0015611
Specht, C. G. et al. Quantitative nanoscopy of inhibitory synapses: counting gephyrin molecules and receptor binding sites. Neuron 79, 308–321 (2013)10.1016/j.neuron.2013.05.013
Li, F. et al. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat. Struct. Mol. Biol. 14, 890–896 (2007)10.1038/nsmb1310
Arora, P. S., Ansari, A. Z., Best, T. P., Ptashne, M. & Dervan, P. B. Design of artificial transcriptional activators with rigid poly-L-proline linkers. J. Am. Chem. Soc. 124, 13067–13071 (2002)ja0208355[pii]
Boncompain, G. et al. Synchronization of secretory protein traffic in populations of cells. Nat. Methods 9, 493–498 (2012)10.1038/nmeth.1928
Hogan, P. G., Lewis, R. S. & Rao, A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 28, 491–533 (2010)10.1146/annurev.immunol.021908.132550
Cahalan, M. D. STIMulating store-operated Ca(2+) entry. Nat. Cell Biol. 11, 669–677 (2009)10.1038/ncb0609-669
Palmer, A. E., Jin, C., Reed, J. C. & Tsien, R. Y. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl Acad. Sci. USA 101, 17404–17409 (2004)10.1073/pnas.0408030101
Miao, Y. et al. An essential and NSF independent role for alpha-SNAP in store-operated calcium entry. eLife 2, e00802 (2013).doi:10.7554/eLife.00802
Giordano, F. et al. PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell 153, 1494–1509 (2013)10.1016/j.cell.2013.05.026
Manford, A. G., Stefan, C. J., Yuan, H. L., Macgurn, J. A. & Emr, S. D. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev. Cell 23, 1129–1140 (2012)10.1016/j.devcel.2012.11.004
Peretti, D., Dahan, N., Shimoni, E., Hirschberg, K. & Lev, S. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol. Biol. Cell 19, 3871–3884 (2008)10.1091/mbc.E08-05-0498
Amarilio, R., Ramachandran, S., Sabanay, H. & Lev, S. Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J. Biol. Chem. 280, 5934–5944 (2005)10.1074/jbc.M409566200
Boss, W. F. & Im, Y. J. Phosphoinositide signaling. Annu. Rev. Plant Biol. 63, 409–429 (2012)10.1146/annurev-arplant-042110-103840
Andersen, O. S. & Koeppe, R. E. 2nd Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 36, 107–130 (2007)10.1146/annurev.biophys.36.040306.132643
Domanska, M. K., Kiessling, V. & Tamm, L. K. Docking and fast fusion of synaptobrevin vesicles depends on the lipid compositions of the vesicle and the acceptor SNARE complex-containing target membrane. Biophys. J. 99, 2936–2946 (2010)10.1016/j.bpj.2010.09.011
Henley, J. & Poo, M. M. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 14, 320–330 (2004)10.1016/j.tcb.2004.04.006
Chen, J. L. et al. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat. Neurosci. 14, 587–594 (2011)10.1038/nn.2799
Lee, W. C. et al. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol. 4, e29 (2006)10.1371/journal.pbio.0040029
Zhang, D., Vjestica, A. & Oliferenko, S. Plasma membrane tethering of the cortical ER necessitates its finely reticulated architecture. Curr. Biol. 22, 2048–2052 (2012)10.1016/j.cub.2012.08.047
D’Angelo, G. et al. Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature 501, 116–120 (2013)10.1038/nature12423
Levine, T. & Rabouille, C. Endoplasmic reticulum: one continuous network compartmentalized by extrinsic cues. Curr. Opin. Cell Biol. 17, 362–368 (2005)10.1016/j.ceb.2005.06.005
Prinz, W. A. Lipid trafficking sans vesicles: where, why, how? Cell 143, 870–874 (2010)10.1016/j.cell.2010.11.031
De Saint-Jean, M. et al. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J. Cell Biol. 195, 965–978 (2011)10.1083/jcb.201104062
Galli, T. et al. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol. Biol. Cell 9, 1437–1448 (1998).
Mallard, F. et al. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156, 653–664 (2002)10.1083/jcb.200110081
Muzerelle, A. et al. Tetanus neurotoxin-insensitive vesicle-associated membrane protein localizes to a presynaptic membrane compartment in selected terminal subsets of the rat brain. Neurosci. 122, 59–75 (2003).
Cebrian, I. et al. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell 147, 1355–1368 (2011)10.1016/j.cell.2011.11.021
Pranke, I. M. et al. α-Synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding. J. Cell Biol. 194, 89–103 (2011)10.1083/jcb.201011118
Vojtek, A. B. & Hollenberg, S. M. Ras-Raf interaction: two-hybrid analysis. Methods Enzymol. 255, 331–342 (1995).
Fromont-Racine, M., Rain, J. C. & Legrain, P. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat. Genet. 16, 277–282 (1997)10.1038/ng0797-277
Lohse, K. et al. Axonal origin and purity of growth cones isolated from fetal rat brain. Brain Res. Dev. Brain Res. 96, 83–96 (1996).
Pfenninger, K. H., Ellis, L., Johnson, M. P., Friedman, L. B. & Somlo, S. Nerve growth cones isolated from fetal rat brain: subcellular fractionation and characterization. Cell 35, 573–584 (1983).
Ellis, L., Katz, F. & Pfenninger, K. H. Nerve growth cones isolated from fetal rat brain. II. Cyclic adenosine 3’:5’-monophosphate (cAMP)-binding proteins and cAMP-dependent protein phosphorylation. J. Neurosci. 5, 1393–1401 (1985).
Tareste, D., Shen, J., Melia, T. J. & Rothman, J. E. SNAREpin/Munc18 promotes adhesion and fusion of large vesicles to giant membranes. Proc. Natl Acad. Sci. USA 105, 2380–2385 (2008)10.1073/pnas.0712125105
Swift, L. L. Assembly of very low density lipoproteins in rat liver: a study of nascent particles recovered from the rough endoplasmic reticulum. J. Lipid Res. 36, 395–406 (1995).
Van Meer, G. & de Kroon, A. I. Lipid map of the mammalian cell. J. Cell Sci. 124, 5–8 (2011)10.1242/jcs.071233
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Ji, H. et al. Protein determinants of SNARE-mediated lipid mixing. Biophys. J. 99, 553–560 (2010)10.1016/j.bpj.2010.04.060
Rothbauer, U. et al. A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol. Cell. Proteom. 7, 282–289 (2008)10.1074/mcp.M700342-MCP200
Saito, T. In vivo electroporation in the embryonic mouse central nervous system. Nat. Protocols 1, 1552–1558 (2006)10.1038/nprot.2006.276
Izeddin, I. et al. Wavelet analysis for single molecule localization microscopy. Opt. Express 20, 2081–2095 (2012).
El Beheiry, M. & Dahan, M. ViSP: representing single-particle localizations in three dimensions. Nat. Methods 10, 689–690 (2013)10.1038/nmeth.2566
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005)10.1016/j.jsb.2005.07.007
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996)10.1006/jsbi.1996.0013
Acknowledgements
We are grateful to F. Polleux, V. Medvedeva and A. Assali for advice on in utero electroporation, N. Rouach for calcium imaging and F. Perez for the gift of RUSH reagents. We are grateful to Y. N. Jan for support to M.P. in revising the manuscript, and L. Bosanac for support with software. We are grateful to A. Pierani and members of the Galli laboratory for discussions. We acknowledge France-BioImaging infrastructure supported by the French National Research Agency (ANR-10-INSB-04, ‘Investments for the future’). Work in our group was financially supported in part by grants from INSERM, CNRS, the Association Française contre les Myopathies (AFM), the Association pour la Recherche sur le Cancer (ARC), the Mairie de Paris Medical Research and Health Program, the Fondation pour la Recherche Médicale (FRM), the Ecole des Neurosciences de Paris (ENP), and awards of the Association Robert Debré pour la Recherche Médicale to T.G., ANR JC grant to D.T. and the Ecole des Neurosciences de Paris (ENP) and award of the Fondation des Treilles to M.P. F.D. is supported by a PhD fellowship from Paris Descartes University and funds by the PhD Program Ecole Doctorale Frontières du Vivant (FdV)—Programme Bettencourt.
Author information
Authors and Affiliations
Contributions
M.P., C.L.J. and T.G. conceived the project. M.P. generated and analysed data in mammalian cells and neurons, and A.J. in budding yeast. F.D. and D.T. performed and analysed liposome fusion assays. M.P., C.G.S. and I.I. performed and C.G.S and I.I analysed results from super-resolution microscopy; X.D. and A.T. set-up the super-resolution microscopes. M.P. and K.H.P. performed and analysed subcellular fractionation experiments. D.V. and J-M.V. generated and analysed electron microscopy data. M.P., C.L.J. and T.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1
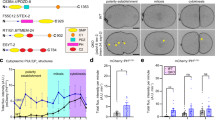
(a) Immunochemistry of VAMP4 and Ykt6 in mouse cortical neurons, further showing observed strong growth cone enrichment is not a general characteristic of R-SNAREs, but specific to a subset of them (Sec22b, VAMP2 and TI-VAMP). VAMP4 is abundant in the perikaryon, but sparsely present in the neurites, while Ykt6 is clearly detectable in neurites as well as perikaryon, but does not have strong localization in the growth cones. Scale bar 10 μm. (b) Impact of Sec22b knockdown in vivo on the distribution of neurons between subdivisions of cortex. In utero electroporation in wild type mouse brains was performed with shRNA1, shRNA2 or scrambled construct as control (scrambled: n = 13 brain sections from 3 embryos; shRNA1: n = 3 brain sections from 2 embryos; shRNA2: n = 9 brain sections from 3 embryos), co-electoporated with GFP under CAG promoter. Lower panel: Brain sections with similar targeting of the electroporation in the same coronal plane were subdivided into regions based on DAPI staining: cortical plate (CP), IZ (intermediate zone) and SVZ/VZ (subventricular/ventricular zone) (first row). Selected regions were transferred to threshold images of the GFP signal (second row), where GFP-positive neurons were outlined (third to fifth row) and measured with Analyze particle tool in ImageJ, using the same parameters for all images. Upper panel: GFP+ positive cells in each subdivision were expressed as percentage of total number of GFP+ cells ± s.e.m. One way Anova showed no significant difference between conditions for all three regions. P ≥ 0.5 n.s. non-significant. Scale bar 100μm.
Supplementary Figure 2
(a) SDS-PAGE gel of R-SNARE (Sec22b) and Q-SNARE (co-expressed Stx1/SNAP25, separately expressed Stx1+SNAP25 or Stx1 alone) liposomes used in the lipid mixing assay (Fig. 3g, h). Separately expressed Q-SNARE was formed by incubating Stx1 liposomes overnight on ice with SNAP25 in a 1:3 (Stx1:SNAP25) molar ratio followed by isolation on a Nycodenz flotation gradient. The smaller size of Stx1 in the case of co-expressed Q-SNAREs is due to the absence of His6-tag. Lipids can be seen at the bottom of the gel. (b) Upper row: Immunolocalization of GFP-Sec22b with a 33 proline linker and calreticulin antibody in cortical neurons. Scale bar: 10 μm, inset: 2 μm. Lower row: COS7 cells co-transfected with GFP-Sec22b containing 33 proline linker and RFP-Sec22b. (c) Immunoprecipitation of GFP-Sec22b with 33 proline linker. COS7 cells were transfected with mCherry-Stx1 and GFP-Sec22b or the GFP-Sec22b with 33 proline linker. Immunoprecipitation was performed with mouse Stx1 antibody, mouse GFP antibody or mouse immunoglobulins as negative control of specificity of immunoprecipitation. Western blot was revealed with rabbit GFP and syntaxin 1 antibodies. mCherry-Stx1 co-immunoprecipitated both GFP-Sec22b variants and vice versa. (d, e) RUSH secretory assay. HeLa cells were co-transfected with GFP-Sec22b or GFP-Sec22bP33 and mCherry tagged VSV-G RUSH construct blocked in the ER. Secretion is released with addition of biotin, and cells are fixed after 30min (n = 76 cells for WT; n = 96 cells for P33), 60min (n = 92 for WT; n = 99 for P33) and 6h (n = 72 for WT; n = 71 for P33). Amount of surface VSV-G was evaluated by surface staining and expressed as ratio to total VSV-G, and normalized to the negative control (n = 77 for WT; n = 72 for P33), which are cells never exposed to biotin (t = 0 min). (d) Representative images are presented, with scale bar 10 μm. (e) Mean value per condition and time point are shown, where error bars represent the s.e.m. Student t-test did not reveal any significant difference between the WT and P33 Sec22b constructs at any of the time points measured. P ≥ 0,05 n.s.
Supplementary Figure 3
(a) Growth defect of the temperature sensitive sec22-3 yeast strain is rescued by expression of GFP-Sec22p. RSY279 sec22-3 cells expressing GFP-Sec22 from a low-copy centromeric plasmid (pCM188-GFP-Sec22) were serially diluted onto medium selective for the plasmid and grown at 24 °C (permissive) and at 30 °C and 37 °C (restrictive temperatures) for 2 days. (b) Correct PM localization of GFP-Sso1 in wild type yeast cells. BY4742 GFP-Sso1 expressing cells in exponential growth were imaged by confocal microscopy at 24 °C; similar results were obtained for wild type cells (SEY6210). Scale bar 3 μm. (c) Sac1 (the PI4P phosphatase acting on the PM PI4P pool) is correctly localized in sec22-3. RSY279 sec22-3 cells expressing GFP-Sac1 were incubated for 0, 30 or 60 minutes at 37 °C, then imaged; representative images show normal ER localization of Sac1 at both time points after shift to restrictive temperature.
Supplementary Figure 4
Uncropped Western blots referring to main figures 1.a, 1.b, 3.b-f. Regions shown in main figures are marked with red squares. All Western blots were named according to the main figure to which they relate to. (Fig. 1a) Distribution analysis of growth cone fractionation showing specific enrichment of GAP43, axonal growth cone marker. (Fig. 1b) Enrichment of R-SNAREs in growth cone membrane fraction compared to total brain homogenate. GAP43 and nucleoporins were used as positive and negative control, respectively. (Fig. 3b) Western blot showing Stx1 immunoprecipitation from embryonic and adult brain, blotted for Stx1 on the left, and reblotted on the same membrane for Sec22b on the right. (Fig. 3c) Enrichment of SNAPs in growth cone membrane fraction compared to total brain homogenate. (Fig. 3d) Western blot showing SNAP25 immunoprecipitation from embryonic and adult brain, blotted for SNAP25 and VAMP2, and Sec22b. Membrane on the left shows SNAP25 and VAMP2, and membrane on the right from a separate gel done in the same way shows SNAP-25 and Sec22b. (Fig. 3e) Western blot showing SNAP23 immunoprecipitation from embryonic and adult brain, blotted for SNAP23 and VAMP2 on the left, and reblotted on the same membrane for Sec22b on the right. (Fig. 3f) Western blot showing SNAP29 immunoprecipitation from embryonic and adult brain, blotted for Stx1, Sec 22b and VAMP2 on the left, and membrane on the right from a separate gel done in the same way shows SNAP-29.
Supplementary Figure 5
Uncropped Western blots referring to main figures 6.a-c, 6.e. Regions shown in main figures are marked with red squares. All Western blots were named according to the main figure to which they relate for simplicity. (Fig. 6a) Western blot showing GFP-Sso1 and GFP-Sec22p immunoprecipitation from yeast, blotted for GFP, Sso1 and Sec22p. (Fig. 6b) Western blot showing GFP-Sso1 expression over time from plasmid under tetracycline-repressible promoter (pAP87-GFP-Sso1) stimulated with doxycycline. Membrane is blotted for Sec22. (Fig. 6c) Western blot showing GFP immunoprecipitation from GFP-Sso1 and GFP-Vam7 expressing yeast cells, blotted for Sec22. (Fig. 6e) Western blot showing GFP immunoprecipitation from GFP-Osh2 and GFP-Osh3 expressing yeast cells, blotted for GFP, Sso1 and Sec22p.
Supplementary information
Supplementary Information
Supplementary Information (PDF 427 kb)
Supplementary Table 1
Supplementary Information (XLSX 14 kb)
Sec22b colocalizes with ER marker in COS7 cells.
Videomicroscopy of COS7 cells co-transfected with mCherry-Sec22b and GFP-Sec61 reveals colocalization of the two constructs and clear ER morphology of the Sec22b compartment. Addition to (Fig. 1g). (AVI 127 kb)
Sec22b compartment has tubule-reticular morphology in neurites.
Videomicroscopy of DIV2 mouse cortical neurons transfected with EGFP-Sec22b reveals clear tubulo-reticular morphology of the Sec22b compartment. Addition to (Fig. 1g). (AVI 986 kb)
Sec22b tubules enter growth cone filopodia.
Confocal microscopy of DIV2 mouse cortical neurons transfected with EGFP-Sec22b reveals active dynamic of Sec22b tubules. Addition to (Fig. 1g). (AVI 560 kb)
Rights and permissions
About this article
Cite this article
Petkovic, M., Jemaiel, A., Daste, F. et al. The SNARE Sec22b has a non-fusogenic function in plasma membrane expansion. Nat Cell Biol 16, 434–444 (2014). https://doi.org/10.1038/ncb2937
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2937