Abstract
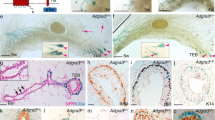
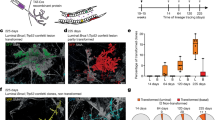
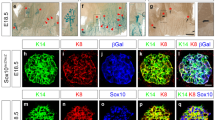
Notch signalling is implicated in stem and progenitor cell fate control in numerous organs. Using conditional in vivo genetic labelling we traced the fate of cells expressing the Notch2 receptor paralogue and uncovered the existence of two previously unrecognized mammary epithelial cell lineages that we term S (Small) and L (Large). S cells appear in a bead-on-a-string formation and are embedded between the luminal and basal/myoepithelial layers in a unique reiterative pattern, whereas single or paired L cells appear among ductal and alveolar cells. Long-term lineage tracing and functional studies indicate that S and L cells regulate ipsi- and contralateral spatial placement of tertiary branches and formation of alveolar clusters. Our findings revise present models of mammary epithelial cell hierarchy, reveal a hitherto undescribed mechanism regulating branching morphogenesis and may have important implications for identification of the cell-of-origin of distinct breast cancer subtypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Shackleton, M. et al. Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 (2006).
Asselin-Labat, M. L. et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 9, 201–209 (2007).
Stingl, J. et al. Purification and unique properties of mammary epithelial stem cells. Nature 439, 993–997 (2006).
Stingl, J., Raouf, A., Eirew, P. & Eaves, C. J. Deciphering the mammary epithelial cell hierarchy. Cell Cycle 5, 1519–1522 (2006).
Sleeman, K. E., Kendrick, H., Ashworth, A., Isacke, C. M. & Smalley, M. J. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res 8, R7 (2006).
Villadsen, R. et al. Evidence for a stem cell hierarchy in the adult human breast. J. Cell Biol. 177, 87–101 (2007).
Visvader, J. E. & Lindeman, G. J. The unmasking of novel unipotent stem cells in the mammary gland. EMBO J. 30, 4858–4859 (2011).
Van Keymeulen, A. et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature 479, 189–193 (2011).
Artavanis-Tsakonas, S. & Muskavitch, M. A. Notch: the past, the present, and the future. Curr. Top. Dev. Biol. 92, 1–29 (2010).
Politi, K. et al. Notch in mammary gland development and breast cancer. Semin. Cancer Biol. 5, 341–7 (2004).
Chiba, S. Notch signaling in stem cell systems. Stem Cells 24, 2437–2447 (2006).
Bouras, T. et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 3, 429–441 (2008).
Buono, K. D. et al. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev. Biol. 293, 565–580 (2006).
Raouf, A. et al. Transcriptome analysis of the normal human mammary cell commitment and differentiation process. Cell Stem Cell 3, 109–118 (2008).
Yalcin-Ozuysal, O. et al. Antagonistic roles of Notch and p63 in controlling mammary epithelial cell fates. Cell Death Differ. 17, 1600–1612 (2010).
Raafat, A. et al. Expression of Notch receptors, ligands, and target genes during development of the mouse mammary gland. J. Cell. Physiol. 226, 1940–1952 (2011).
O’Neill, C. F. et al. Notch2 signaling induces apoptosis and inhibits human MDA-MB-231 xenograft growth. Am. J. Pathol. 171, 1023–1036 (2007).
Parr, C., Watkins, G. & Jiang, W. G. The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancer. Int. J. Mole. Med. 14, 779–786 (2004).
Florena, A. M. et al. Associations between Notch-2, Akt-1 and HER2/neuexpression in invasive human breast cancer: a tissue microarray immunophenotypic analysis on 98 patients. Pathobiol.: J. Immunopathol. Mol. Cell. Biol. 74, 317–322 (2007).
Thomas, G. et al. A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1). Nat. Gene. 41, 579–584 (2009).
Fu, Y. P. et al. NOTCH2 in breast cancer: association of SNP rs11249433 with gene expression in ER-positive breast tumors without TP53 mutations. Mol. Cancer 9, 113 (2010).
Robinson, D. R. et al. Functionally recurrent rearrangements of theMAST kinase and Notch gene families in breast cancer. Nat. Med. 17, 1646–1651 (2011).
Fre, S. et al. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PloS One 6, e25785 (2011).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Gene. 21, 70–71 (1999).
Saito, M. et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat. Biotechnol. 19, 746–750 (2001).
Chen, L. H. & Bissell, M. J. A novel regulatory mechanism for whey acidic protein gene expression. Cell Regul. 1, 45–54 (1989).
Keller, P. J., Arendt, L. M. & Kuperwasser, C. Stem cell maintenance of the mammary gland: it takes two. Cell Stem Cell 9, 496–497 (2011).
Visvader, J. E. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Gene. Dev. 23, 2563–2577 (2009).
Simian, M. et al. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 128, 3117–3131 (2001).
Wiseman, B. S. et al. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J. Cell Biol. 162, 1123–1133 (2003).
Sakai, T., Larsen, M. & Yamada, K. M. Fibronectin requirement in branching morphogenesis. Nature 423, 876–881 (2003).
Nelson, C. M., Vanduijn, M. M., Inman, J. L., Fletcher, D. A. & Bissell, M. J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314, 298–300 (2006).
Sternlicht, M. D., Kouros-Mehr, H., Lu, P. & Werb, Z. Hormonal and local control of mammary branching morphogenesis. Diff.; Res. Biol. Diversity 74, 365–381 (2006).
Wagner, K. U. et al. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development 129, 1377–1386 (2002).
Oakes, S. R. et al. The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Gene. Dev. 22, 581–586 (2008).
Oliver, C. H., Khaled, W. T., Frend, H., Nichols, J. & Watson, C. J. The Stat6-regulated KRAB domain zinc finger protein Zfp157 regulates the balance of lineages in mammary glands and compensates for loss of Gata-3. Gene. Dev. 26, 1086–1097 (2012).
Smith, G. H. & Chepko, G. Mammary epithelial stem cells. Microsc. Res. Techn. 52, 190–203 (2001).
Acknowledgements
The authors would like to thank J. S. Brugge for her long-standing and very generous technical and theoretical advice throughout this project as well as for critical reading of the manuscript. We thank A. Shiang-Ru Kaanta for technical advice and critical reading of the manuscript and also A. Louvi for critical reading of the manuscript. In addition, we would also like to thank the Nikon Imaging Center at Harvard Medical School for help with light microscopy and the Animal Facility at Harvard Center for Comparative Medicine for helping in the maintenance and care of the transgenic animals. This work was supported by grants from the NIH to S.A-T.
Author information
Authors and Affiliations
Contributions
S.S. conceptualized, designed and performed all experiments and data analysis; D.L. contributed to transplantation experiments; S.A-T. conceived the study. S.S. and S.A-T. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 957 kb)
Supplementary Information
Supplementary Table 1 (XLS 13 kb)
S Cell String.
This video is related to Supplementary Fig. S3b. (AVI 3954 kb)
Rights and permissions
About this article
Cite this article
Šale, S., Lafkas, D. & Artavanis-Tsakonas, S. Notch2 genetic fate mapping reveals two previously unrecognized mammary epithelial lineages. Nat Cell Biol 15, 451–460 (2013). https://doi.org/10.1038/ncb2725
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2725
This article is cited by
-
Transcription factor FoxO1 regulates myoepithelial cell diversity and growth
Scientific Reports (2024)
-
The mutational landscape of the adult healthy parous and nulliparous human breast
Nature Communications (2023)
-
Role of Snai2 and Notch signaling in salivary gland myoepithelial cell fate
Laboratory Investigation (2022)
-
Mammary Development and Breast Cancer: a Notch Perspective
Journal of Mammary Gland Biology and Neoplasia (2021)
-
The Notch system during pubertal development of the bovine mammary gland
Scientific Reports (2019)