Abstract
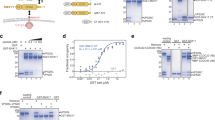
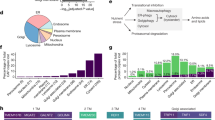
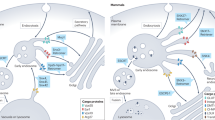
The PDZ-domain-containing sorting nexin 27 (SNX27) promotes recycling of internalized transmembrane proteins from endosomes to the plasma membrane by linking PDZ-dependent cargo recognition to retromer-mediated transport. Here, we employed quantitative proteomics of the SNX27 interactome and quantification of the surface proteome of SNX27- and retromer-suppressed cells to dissect the assembly of the SNX27 complex and provide an unbiased global view of SNX27-mediated sorting. Over 100 cell surface proteins, many of which interact with SNX27, including the glucose transporter GLUT1, the Menkes disease copper transporter ATP7A, various zinc and amino acid transporters, and numerous signalling receptors, require SNX27–retromer to prevent lysosomal degradation and maintain surface levels. Furthermore, we establish that direct interaction of the SNX27 PDZ domain with the retromer subunit VPS26 is necessary and sufficient to prevent lysosomal entry of SNX27 cargo. Our data identify the SNX27–retromer as a major endosomal recycling hub required to maintain cellular nutrient homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
23 April 2013
In the version of this Article originally published, labels were incorrectly aligned to the blots in Fig. 6c,d. Furthermore, the middle initial of the author Elaine C. Thomas was missing.
04 July 2014
In the version of this Article originally published, an error in the journal production process led to the wrong image being used for 'SNX27KD' in Fig. 4b. The image has been corrected in all online versions of the Article.
01 August 2014
Nat. Cell Biol. 15, 461–471 (2013); published online 7 April 2013; corrected after print 4 July 2014 In the version of this Article originally published, an error in the journal production process led to the wrong image being used for 'SNX27KD' in Fig. 4b. The correct image is shown below and is corrected in all online versions of the Article.
References
Cullen, P. J. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat. Rev. Mol. Cell Biol. 9, 574–582 (2008).
Van Kerkhof, P. et al. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J. 24, 2851–2861 (2005).
Steinberg, F., Heesom, K. J., Bass, M. D. & Cullen, P. J. SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J. Cell Biol. 197, 219–230 (2012).
Bottcher, R. T. et al. Sorting nexin 17 prevents lysosomal degradation of β1integrins by binding to the β1-integrin tail. Nat. Cell Biol. 14, 584–592 (2012).
Lauffer, B. E. et al. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J. Cell Biol. 190, 565–574 (2010).
Temkin, P. et al. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat. Cell Biol. 13, 715–721 (2011).
Arighi, C. N., Hartnell, L. M., Aguilar, R. C., Haft, C. R. & Bonifacino, J. S. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165, 123–133 (2004).
Carlton, J. et al. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr. Biol. 14, 1791–1800 (2004).
Seaman, M. N. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J. Cell Biol. 165, 111–122 (2004).
Wassmer, T. et al. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J. Cell Sci. 120, 45–54 (2007).
Wassmer, T. et al. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev. Cell 17, 110–122 (2009).
Trinkle-Mulcahy, L. et al. Identifying specific protein interaction partners using quantitative mass spectrometry and bead proteomes. J. Cell Biol. 183, 223–239 (2008).
Rincon, E. et al. Proteomics identification of sorting nexin 27 as a diacylglycerol kinase zeta-associated protein: new diacylglycerol kinase roles in endocytic recycling. Mol. Cell Proteomics 6, 1073–1087 (2007).
Valdes, J. L. et al. Sorting nexin 27 protein regulates trafficking of a p21-activated kinase (PAK) interacting exchange factor (β-Pix)-G protein-coupled receptor kinase interacting protein (GIT) complex via a PDZ domain interaction. J. Biol. Chem. 286, 39403–39416 (2011).
Balana, B. et al. Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27. Proc. Natl Acad. Sci. USA 108, 5831–5836 (2011).
Setty, S. R. et al. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature 454, 1142–1146 (2008).
Zhao, F. Q. & Keating, A. F. Functional properties and genomics of glucose transporters. Curr. Genom. 8, 113–128 (2007).
Traer, C. J. et al. SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat. Cell Biol. 9, 1370–1380 (2007).
Ghai, R. et al. Phox homology band 4.1/ezrin/radixin/moesin-like proteins function as molecular scaffolds that interact with cargo receptors and Ras GTPases. Proc. Natl Acad. Sci. USA 108, 7763–7768 (2011).
Harbour, M. E., Breusegem, S. Y. & Seaman, M. N. Recruitment of the endosomal WASH complex is mediated by the extended ’tail’ of Fam21 binding to the retromer protein Vps35. Biochem. J. 442, 209–220 (2012).
Jia, D., Gomez, T. S., Billadeau, D. D. & Rosen, M. K. Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer. Mol. Biol. Cell 23, 2352–2361 (2012).
Harbour, M. E. et al. The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J. Cell Sci. 123, 3703–3717 (2010).
Shi, H., Rojas, R., Bonifacino, J. S. & Hurley, J. H. The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat. Struct. Mol. Biol. 13, 540–548 (2006).
Hayashi, H. et al. Sorting nexin 27 interacts with multidrug resistance-associated protein 4 (MRP4) and mediates internalization of MRP4. J. Biol. Chem. 287, 15054–15065 (2012).
Seaman, M. N., Harbour, M. E., Tattersall, D., Read, E. & Bright, N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J. Cell Sci. 122, 2371–2382 (2009).
Elkins, J. M. et al. Structure of PICK1 and other PDZ domains obtained with the help of self-binding C-terminal extensions. Protein Sci. 16, 683–694 (2007).
Bunn, R. C., Jensen, M. A. & Reed, B. C. Protein interactions with the glucose transporter binding protein GLUT1CBP that provide a link between GLUT1 and the cytoskeleton. Mol. Biol. Cell 10, 819–832 (1999).
Wieman, H. L. et al. An essential role for the Glut1 PDZ-binding motif in growth factor regulation of Glut1 degradation and trafficking. Biochem. J. 418, 345–367 (2009).
Puthenveedu, M. A. et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 143, 761–773 (2010).
Gomez, T. S., Gorman, J. A., Artal-Martinez de Narvajas, A., Koenig, A. O. & Billadeau, D. D. Trafficking defects in WASH-knockout fibroblasts originate from collapsed endosomal and lysosomal networks. Mol. Biol. Cell 23, 3215–3228 (2012).
Ghai, R. et al. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc. Natl Acad. Sci. USA 19, E643-52 (2013).
Zech, T. et al. The Arp2/3 activator WASH regulates α5β1-integrin-mediated invasive migration. J. Cell Sci. 124, 3753–3759 (2011).
Acknowledgements
F.S. is a Royal Society Newton Fellow. M.G. and A.J.B. are supported by Wellcome Trust 4 Year PhD Studentships awarded through the Dynamic Cell Biology programme (083474). P.J.C. is supported by the Wellcome Trust (089928 and 085743) and the Royal Society. We thank M. Bass and D. Stephens for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
F.S., J.M.T. and P.J.C. designed the project; F.S., M.G., E.T. and A.J.B. performed the experiments; M.W. performed the bioinformatics; K.J.H. performed the proteomics; F.S., M.G. and P.J.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1908 kb)
Supplementary Table 1
Supplementary Information (XLSX 200 kb)
Supplementary Table 2
Supplementary Information (XLSX 21 kb)
Supplementary Table 3
Supplementary Information (XLSX 75 kb)
Supplementary Table 4
Supplementary Information (XLSX 25 kb)
Supplementary Table 5
Supplementary Information (XLSX 394 kb)
Supplementary Table 6
Supplementary Information (XLSX 10 kb)
Supplementary Table 7
Supplementary Information (XLS 61 kb)
Rights and permissions
About this article
Cite this article
Steinberg, F., Gallon, M., Winfield, M. et al. A global analysis of SNX27–retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol 15, 461–471 (2013). https://doi.org/10.1038/ncb2721
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2721
This article is cited by
-
Partial loss of Sorting Nexin 27 resembles age- and Down syndrome-associated T cell dysfunctions
Immunity & Ageing (2024)
-
VPS35 promotes gastric cancer progression through integrin/FAK/SRC signalling-mediated IL-6/STAT3 pathway activation in a YAP-dependent manner
Oncogene (2024)
-
Sleep fragmentation exacerbates myocardial ischemia‒reperfusion injury by promoting copper overload in cardiomyocytes
Nature Communications (2024)
-
LRP10 and α-synuclein transmission in Lewy body diseases
Cellular and Molecular Life Sciences (2024)
-
ITRAQ-based quantitative proteomic analysis reveals that VPS35 promotes the expression of MCM2-7 genes in HeLa cells
Scientific Reports (2022)