Abstract
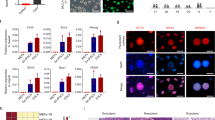
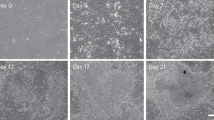
Epiblast stem cells (EpiSCs) derived from epiblast tissue of post-implantation embryos are pluripotent and can give rise to all three germ layers in teratoma assays1,2. Introduction of the four transcription factors Oct4, Sox2, Klf4 and c-Myc into somatic cells has been shown to generate induced pluripotent stem cells (iPSCs) that are very similar to embryonic stem cells (ESCs) in a number of characteristics3,4,5,6. However, generation of EpiSCs by the direct reprogramming of somatic cells using these transcription factors has not been shown to date. Here, we show that these transcription factors can be used to directly generate induced EpiSCs (iEpiSCs) under EpiSC culture conditions. iEpiSCs resemble EpiSCs in morphology, gene expression pattern, epigenetic status and chimaera-forming capability. This study demonstrates that the culture environment in transcription factor-mediated reprogramming determines the cell fate of the reprogrammed cell. We therefore hypothesize that it will eventually be possible to shape the identity of a directly reprogrammed cell simply by modulating culture conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Brons, I. G. et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 (2007).
Tesar, P. J. et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 (2007).
Maherali, N. et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1, 55–70 (2007).
Okita, K., Ichisaka, T. & Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 (2007).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Wernig, M. et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 (2007).
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981).
Gardner, R. L. & Rossant, J. Investigation of the fate of 4–5 day post-coitum mouse inner cell mass cells by blastocyst injection. J. Embryol. Exp. Morphol. 52, 141–152 (1979).
Nakagawa, M. et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26, 101–106 (2008).
Nichols, J. & Smith, A. Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 (2009).
Rossant, J. Stem cells and early lineage development. Cell 132, 527–531 (2008).
Guo, G. et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063–1069 (2009).
Sridharan, R. et al. Role of the murine reprogramming factors in the induction of pluripotency. Cell 136, 364–377 (2009).
Greber, B. et al. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell 6, 215–226 (2010).
Xu, C. et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 19, 971–974 (2001).
Yeom, Y. I. et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 122, 881–894 (1996).
Bao, S. et al. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature 461, 1292–1295 (2009).
Chou, Y. F. et al. The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell 135, 449–461 (2008).
Johansson, B. M. & Wiles, M. V. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol 15, 141–151 (1995).
Yao, S. et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc. Natl Acad. Sci. USA 103, 6907–6912 (2006).
Hadjantonakis, A. K., Gertsenstein, M., Ikawa, M., Okabe, M. & Nagy, A. Non-invasive sexing of preimplantation stage mammalian embryos. Nat. Genet. 19, 220–222 (1998).
Han, D. W. et al. Epiblast stem cell subpopulations represent mouse embryos of distinct pregastrulation stages. Cell 143, 617–627 (2010).
Silva, J. et al. Nanog is the gateway to the pluripotent ground state. Cell 138, 722–737 (2009).
Hayashi, K., Lopes, S. M., Tang, F. & Surani, M. A. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell 3, 391–401 (2008).
Hanna, J. et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell 4, 513–524 (2009).
Hall, J. et al. Oct4 and LIF/Stat3 additively induce Kruppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell 5, 597–609 (2009).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Han, D. W. et al. Pluripotential reprogramming of the somatic genome in hybrid cells occurs with the first cell cycle. Stem Cells 26, 445–454 (2008).
Acknowledgements
We are indebted to all members of the Schöler laboratory for discussions on the results. We are especially grateful to P. Tesar for providing T9 EpiSCs and A. Malapetsas for editing the manuscript. We are also grateful to M. Sinn for technical assistance and I. M. Verma for providing the pBOB-CAG-iCRE-SD plasmid. This work was supported by funds of the Max Planck Society and the Federal Ministry of Education and Research (BMBF) (Grant 01GN0934).
Author information
Authors and Affiliations
Contributions
D.W.H. designed and performed most experiments and wrote the manuscript. B.G. performed microarray experiments, analysed the data and edited the manuscript. M.J.A. analysed the microarray data. N.T. carried out lentivirus construction and infection. C.B. analysed the gene expression data. G.W. and K.K. performed morula aggregation and the teratoma assay, respectively. M.S. performed FACS analysis. H.S. conceived the experiments and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1288 kb)
Rights and permissions
About this article
Cite this article
Han, D., Greber, B., Wu, G. et al. Direct reprogramming of fibroblasts into epiblast stem cells. Nat Cell Biol 13, 66–71 (2011). https://doi.org/10.1038/ncb2136
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2136