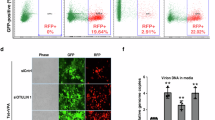
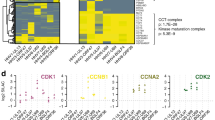
Abstract
The large tegument proteins of herpesviruses encode conserved cysteine proteases of unknown function. Here we show that BPLF1, the Epstein–Barr-virus-encoded member of this protease family, is a deneddylase that regulates virus production by modulating the activity of cullin-RING ligases (CRLs). BPLF1 hydrolyses NEDD8 conjugates in vitro, acts as a deneddylase in vivo, binds to cullins and stabilizes CRL substrates. Expression of BPLF1 alone or in the context of the productive virus cycle induces accumulation of the licensing factor CDT1 and deregulates S-phase DNA synthesis. Inhibition of BPLF1 during the productive virus cycle prevents cellular DNA re-replication and inhibits virus replication. Viral DNA synthesis is restored by overexpression of CDT1. Homologues encoded by other herpesviruses share the deneddylase activity. Thus, these enzymes are likely to have a key function in the virus life cycle by inducing a replication-permissive S-phase-like cellular environment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Glickman, M.H. & Ciechanover, A. The ubiquitin–proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82, 373–428 (2002).
Ciechanover, A., Orian, A. & Schwartz, A. L. The ubiquitin-mediated proteolytic pathway: mode of action and clinical implications. J Cell Biochem Suppl 34, 40–51 (2000).
Reyes-Turcu, F. E., Ventii, K. H. & Wilkinson, K. D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 78, 363–397 (2009).
Kerscher, O., Felberbaum, R. & Hochstrasser, M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22, 159–180 (2006).
Chiba, T. & Tanaka, K. Cullin-based ubiquitin ligase and its control by NEDD8-conjugating system. Curr. Protein Pept. Sci. 5, 177–184 (2004).
Tatham, M. H. et al. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nature Cell Biol. 10, 538–546 (2008).
Lallemand-Breitenbach, V. et al. Arsenic degrades PML or PML-RARα through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nature Cell Biol. 10, 547–555 (2008).
Duda, D. M. et al. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell 134, 995–1006 (2008).
Pan, Z. Q., Kentsis, A., Dias, D. C., Yamoah, K. & Wu, K. Nedd8 on cullin: building an expressway to protein destruction. Oncogene 23, 1985–1997 (2004).
Isaacson, M. K. & Ploegh, H. L. Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe 5, 559–570 (2009).
Loureiro, J. & Ploegh, H. L. Antigen presentation and the ubiquitin–proteasome system in host–pathogen interactions. Adv. Immunol. 92, 225–305 (2006).
Scheffner, M., Huibregtse, J. M., Vierstra, R. D. & Howley, P. M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75, 495–505 (1993).
Boutell, C., Canning, M., Orr, A. & Everett, R. D. Reciprocal activities between herpes simplex virus type 1 regulatory protein ICP0, a ubiquitin E3 ligase, and ubiquitin-specific protease USP7. J. Virol. 79, 12342–12354 (2005).
Saridakis, V. et al. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein–Barr nuclear antigen 1 implications for EBV-mediated immortalization. Mol. Cell 18, 25–36 (2005).
Randow, F. & Lehner, P. J. Viral avoidance and exploitation of the ubiquitin system. Nature Cell Biol. 11, 527–534 (2009).
Young, L. S. & Rickinson, A. B. Epstein–Barr virus: 40 years on. Nature Rev. Cancer 4, 757–768 (2004).
Bishop, G. A. & Busch, L. K. Molecular mechanisms of B-lymphocyte transformation by Epstein–Barr virus. Microbes Infect. 4, 853–857 (2002).
Tsurumi, T., Fujita, M. & Kudoh, A. Latent and lytic Epstein–Barr virus replication strategies. Rev. Med. Virol. 15, 3–15 (2005).
Kudoh, A. et al. Epstein–Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J. Biol. Chem. 280, 8156–8163 (2005).
Sompallae, R. et al. Epstein–Barr virus encodes three bona fide ubiquitin-specific proteases. J. Virol. 82, 10477–10486 (2008).
Kattenhorn, L. M., Korbel, G. A., Kessler, B. M., Spooner, E. & Ploegh, H. L. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell 19, 547–557 (2005).
Mendoza, H. M. et al. NEDP1, a highly conserved cysteine protease that deNEDDylates Cullins. J. Biol. Chem. 278, 25637–25643 (2003).
Borodovsky, A. et al. A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J. 20, 5187–5196 (2001).
Nishitani, H. et al. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 25, 1126–1136 (2006).
Busino, L. et al. Degradation of Cdc25A by β-TrCP during S phase and in response to DNA damage. Nature 426, 87–91 (2003).
Auld, C. A., Fernandes, K. M. & Morrison, R. F. Skp2-mediated p27Kip1 degradation during S/G2 phase progression of adipocyte hyperplasia. J. Cell Physiol. 211, 101–111 (2007).
Ohta, T. & Xiong, Y. Phosphorylation- and Skp1-independent in vitro ubiquitination of E2F1 by multiple ROC-cullin ligases. Cancer Res. 61, 1347–1353 (2001).
Cook, J. G. Replication licensing and the DNA damage checkpoint. Front. Biosci. 14, 5013–5030 (2009).
Wilsker, D. & Bunz, F. Chk1 phosphorylation during mitosis: a new role for a master regulator. Cell Cycle 8, 1161–1163 (2009).
Chen, Y. & Poon, R. Y. The multiple checkpoint functions of CHK1 and CHK2 in maintenance of genome stability. Front. Biosci. 13, 5016–5029 (2008).
Hans, F. & Dimitrov, S. Histone H3 phosphorylation and cell division. Oncogene 20, 3021–3027 (2001).
Guerreiro-Cacais, A. O., Uzunel, M., Levitskaya, J. & Levitsky, V. Inhibition of heavy chain and β2-microglobulin synthesis as a mechanism of major histocompatibility complex class I downregulation during Epstein–Barr virus replication. J. Virol. 81, 1390–1400 (2007).
Daibata, M., Humphreys, R. E., Takada, K. & Sairenji, T. Activation of latent EBV via anti-IgG-triggered, second messenger pathways in the Burkitt's lymphoma cell line Akata. J. Immunol. 144, 4788–4793 (1990).
Chrisp, P. & Clissold, S. P. Foscarnet. A review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs 41, 104–129 (1991).
Yuan, J., Cahir-McFarland, E., Zhao, B. & Kieff, E. Virus and cell RNAs expressed during Epstein–Barr virus replication. J. Virol. 80, 2548–2565 (2006).
Kim, Y. & Kipreos, E. T. Cdt1 degradation to prevent DNA re-replication: conserved and non-conserved pathways. Cell Div. 2, 18 (2007).
Shen, L. N. et al. Structural basis of NEDD8 ubiquitin discrimination by the deNEDDylating enzyme NEDP1. EMBO J. 24, 1341–1351 (2005).
Schlieker, C. et al. Structure of a herpesvirus-encoded cysteine protease reveals a unique class of deubiquitinating enzymes. Mol. Cell 25, 677–687 (2007).
Edelmann, M. J. et al. Structural basis and specificity of human otubain 1-mediated deubiquitination. Biochem. J. 418, 379–390 (2009).
Soucy, T. A. et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458, 732–736 (2009).
Gredmark-Russ, S. et al. A gammaherpesvirus ubiquitin-specific protease is involved in the establishment of murine gammaherpesvirus 68 infection. J. Virol. 83, 10644–10652 (2009).
Bottcher, S. et al. Mutagenesis of the active-site cysteine in the ubiquitin-specific protease contained in large tegument protein pUL36 of pseudorabies virus impairs viral replication in vitro and neuroinvasion in vivo. J. Virol. 82, 6009–6016 (2008).
Jarosinski, K., Kattenhorn, L., Kaufer, B., Ploegh, H. & Osterrieder, N. A herpesvirus ubiquitin-specific protease is critical for efficient T cell lymphoma formation. Proc. Natl Acad. Sci. USA 104, 20025–20030 (2007).
Davy, C. & Doorbar, J. G2/M cell cycle arrest in the life cycle of viruses. Virology 368, 219–226 (2007).
Boehmer, P. E. & Lehman, I. R. Herpes simplex virus DNA replication. Annu. Rev. Biochem. 66, 347–384 (1997).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006).
Hassink, G. C. et al. The ER-resident ubiquitin-specific protease 19 participates in the UPR and rescues ERAD substrates. EMBO Rep. 10, 755–761 (2009).
Zhao, B., Schlesiger, C., Masucci, M. G. & Lindsten, K. The ubiquitin specific protease 4 (Usp4) is a new player in the Wnt signalling pathway. J. Cell. Mol. Med. 13, 1886–1895 (2009).
Duda, D. M. et al. Structure of a SUMO-binding-motif mimic bound to Smt3p–Ubc9p: conservation of a non-covalent ubiquitin-like protein-E2 complex as a platform for selective interactions within a SUMO pathway. J. Mol. Biol. 369, 619–630 (2007).
Yao, G. Q., Grill, S., Egan, W. & Cheng, Y. C. Potent inhibition of Epstein–Barr virus by phosphorothioate oligodeoxynucleotides without sequence specification. Antimicrob. Agents Chemother. 37, 1420–1425 (1993).
Acknowledgements
We thank Ron T. Hay, Frauke Melchior, Zhen-Quian-Pan, Dong-Er Zhang, Chiba Chikatumi and Jürgen Haas for providing plasmids, antibodies and technical advice. This study was supported by grants awarded by the Swedish Cancer Society, the Swedish Medical Research Council, the Karolinska Institutet, Stockholm, Sweden, and by the European Community Integrated Project INCA, contract no. LSHC-CT-2005-018704, and Network of Excellence RUBICON, contract no. LSG-CT-2005-018683. S.H. is supported by a fellowship from the European Community Marie Curie Early Training Network UbiRegulators contract no. MRTN-CT-2006-034555.
Author information
Authors and Affiliations
Contributions
S.G. and M.G.M. designed the experiments; O.F. produced the recombinant lentiviruses; S.H., M.P and C.D.G. performed analysis; S.C. performed bioinformatics analysis; S.G. and M.G.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2223 kb)
Rights and permissions
About this article
Cite this article
Gastaldello, S., Hildebrand, S., Faridani, O. et al. A deneddylase encoded by Epstein–Barr virus promotes viral DNA replication by regulating the activity of cullin-RING ligases. Nat Cell Biol 12, 351–361 (2010). https://doi.org/10.1038/ncb2035
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2035