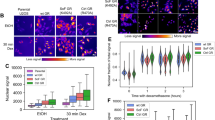
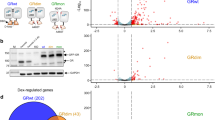
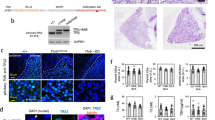
Abstract
Studies on glucocorticoid receptor (GR) action typically assess gene responses by long-term stimulation with synthetic hormones. As corticosteroids are released from adrenal glands in a circadian and high-frequency (ultradian) mode, such treatments may not provide an accurate assessment of physiological hormone action. Here we demonstrate that ultradian hormone stimulation induces cyclic GR-mediated transcriptional regulation, or gene pulsing, both in cultured cells and in animal models. Equilibrium receptor-occupancy of regulatory elements precisely tracks the ligand pulses. Nascent RNA transcripts from GR-regulated genes are released in distinct quanta, demonstrating a profound difference between the transcriptional programs induced by ultradian and constant stimulation. Gene pulsing is driven by rapid GR exchange with response elements and by GR recycling through the chaperone machinery, which promotes GR activation and reactivation in response to the ultradian hormone release, thus coupling promoter activity to the naturally occurring fluctuations in hormone levels. The GR signalling pathway has been optimized for a prompt and timely response to fluctuations in hormone levels, indicating that biologically accurate regulation of gene targets by GR requires an ultradian mode of hormone stimulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lightman, S. L. et al. Hypothalamic-pituitary-adrenal function. Arch. Physiol Biochem. 110, 90–93 (2002).
Lightman, S. L. Patterns of exposure to glucocorticoid receptor ligand. Biochem. Soc. Trans. 34, 1117–1118 (2006).
Droste, S. K. et al. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology 149, 3244–3253 (2008).
Young, E. A., Abelson, J. & Lightman, S. L. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol. 25, 69–76 (2004).
Lewis, J. G., Bagley, C. J., Elder, P. A., Bachmann, A. W. & Torpy, D. J. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin. Chim. Acta 359, 189–194 (2005).
McNally, J. G., Mueller, W. G., Walker, D., Wolford, R. G. & Hager, G. L. The glucocorticoid receptor: Rapid exchange with regulatory sites in living cells. Science 287, 1262–1265 (2000).
Kramer, P. et al. Transcriptional state of the mouse mammary tumor virus promoter can effect topological domain size in vivo. J. Biol. Chem. 274, 28590–28597 (1999).
Walker, D., Htun, H. & Hager, G. L. Using inducible vectors to study intracellular trafficking of GFP-tagged steroid/nuclear receptors in living cells. Methods 19, 386–393 (1999).
Becker, M. et al. Dynamic behavior of transcription factors on a natural promoter in living cells. EMBO Rep. 3, 1188–1194 (2002).
Stavreva, D. A., Muller, W. G., Hager, G. L., Smith, C. L. & McNally, J. G. Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Mol. Cell. Biol. 24, 2682–2697 (2004).
Liu, J. & DeFranco, D. B. Chromatin recycling of glucocorticoid receptors: implications for multiple roles of heat shock protein 90. Mol. Endocrinol. 13, 355–365 (1999).
Qi, M., Stasenko, L. J. & DeFranco, D. B. Recycling and desensitization of glucocorticoid receptors in v- mos transformed cells depend on the ability of nuclear receptors to modulate gene expression. Mol. Endocrinol. 4, 455–464 (1990).
Munck, A. & Foley, R. Kinetics of glucocorticoid-receptor complexes in rat thymus cells. J. Steroid Biochem. 7, 1117–1122 (1976).
Pratt, W. B., Galigniana, M. D., Harrell, J. M. & DeFranco, D. B. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movement. Cell Signal. 16, 857–872 (2004).
Freeman, B. C. & Yamamoto, K. R. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296, 2232–2235 (2002).
Freeman, B. C. & Yamamoto, K. R. Continuous recycling: a mechanism for modulatory signal transduction. Trends Biochem. Sci. 26, 285–290 (2001).
Rayasam, G. V. et al. Ligand specific dynamics of the progesterone receptor in living cells and during chromatin remodeling in vitro. Mol. Cell. Biol. 25, 2406–2418 (2005).
Klokk, T. I. et al. Ligand-specific dynamics of the androgen receptor at its response element in living cells. Mol. Cell Biol. 27, 1823–1843 (2007).
John, S. et al. Interaction of the glucocorticoid receptor with the global chromatin landscape. Mol. Cell 29, 611–624 (2008).
Sugaya, K., Vigneron, M. & Cook, P. R. Mammalian cell lines expressing functional RNA polymerase II tagged with the green fluorescent protein. J. Cell Sci. 113 (Pt 15), 2679–2683 (2000).
Conway-Campbell, B. L. et al. Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology 148, 5470–5477 (2007).
Metivier, R. et al. Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115, 751–763 (2003).
Kang, Z., Pirskanen, A., Janne, O. A. & Palvimo, J. J. Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J. Biol. Chem. 277, 48366–48371 (2002).
Rothman, M. S. & Wierman, M. E. The role of gonadotropin releasing hormone in normal and pathologic endocrine processes. Curr. Opin. Endocrinol. Diabetes Obes. 14, 306–310 (2007).
Thompson, N. M., Davies, J. S., Mode, A., Houston, P. A. & Wells, T. Pattern-dependent suppression of growth hormone (GH) pulsatility by ghrelin and GH-releasing peptide-6 in moderately GH-deficient rats. Endocrinology 144, 4859–4867 (2003).
Chadwick, D. & Goode, J. A. Mechanisms and Biological Significance of Pulsatile Hormone Secretion (John Wiley & Sons Ltd., 2000).
Belchetz, P. E., Plant, T. M., Nakai, Y., Keogh, E. J. & Knobil, E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202, 631–633 (1978).
Gore, A. C. GnRH: The master molecule of reproduction (Kluwer Academic Publishers, 2002).
Geva-Zatorsky, N. et al. Oscillations and variability in the p53 system. Mol. Syst. Biol. 2, 2006 (2006).
Batchelor, E., Mock, C. S., Bhan, I., Loewer, A. & Lahav, G. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol. Cell 30, 277–289 (2008).
Nelson, D. E. et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science 306, 704–708 (2004).
Karpova, T. S. et al. Concurrent fast and slow cycling of a transcriptional activator at an endogenous promoter. Science 319, 466–469 (2008).
Dolmetsch, R. E., Xu, K. & Lewis, R. S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 (1998).
Cai, L., Dalal, C. K. & Elowitz, M. B. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature 455, 485–490 (2008).
Chubb, J. R., Trcek, T., Shenoy, S. M. & Singer, R. H. Transcriptional pulsing of a developmental gene. Curr. Biol. 16, 1018–1025 (2006).
Mezo, G., Manea, M., Szabi, I., Vincze, B. & Kovacs, M. New derivatives of GnRH as potential anticancer therapeutic agents. Curr. Med. Chem. 15, 2366–2379 (2008).
Schacke, H., Docke, W. D. & Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 96, 23–43 (2002).
Sharp, Z. D. et al. Estrogen-receptor-alpha exchange and chromatin dynamics are ligand- and domain-dependent. J Cell Sci. 119, 4101–4116 (2006).
Bosisio, D. et al. A hyper-dynamic equilibrium between promoter-bound and nucleoplasmic dimers controls NF-kB-dependent gene activity. EMBO J. 25, 798–810 (2006).
Hager, G. L. et al. Chromatin dynamics and the evolution of alternate promoter states. Chromosome. Res. 14, 107–116 (2006).
Muller, W. G., Walker, D., Hager, G. L. & McNally, J. G. Large-scale chromatin decondensation and recondensation regulated by transcription from a natural promoter. J. Cell Biol. 154, 33–48 (2001).
Vallone, D. et al. Neoplastic transformation of rat thyroid cells requires the junB and fra-1 gene induction which is dependent on the HMGI-C gene product. EMBO J. 16, 5310–5321 (1997).
Acknowledgements
We thank T. Karpova and J. McNally for assistance, C. Zeiss Inc. for the opportunity to carry out live cell experiments on a Zeiss Duoscan microscope, D. Shatti for technical assistance with nuclear extract preparation and Y. Kershaw for assistance with the in vivo experiments. This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The animal work was supported by a BBSRC grant (BB/C51297X/1) and a Wellcome Trust Programme Grant (074112/Z/04/Z) to S.L. The qPCR was performed on an Applied Biosystems 7500 System funded by a Wellcome Trust Equipment Grant (075548/Z/04/Z).
Author information
Authors and Affiliations
Contributions
D.A.S. designed and performed most of the experiments and, together with G.L.H., wrote the initial draft of the manuscript; M.W. and D.A.S. performed transcription experiments, analysed data and participated in manuscript preparation and data interpretation; M.W. also performed all cell-line ChIP experiments; S.J. and T.A.J. performed confirmatory experiments, designed primers for PCR and participated in data interpretation and manuscript revision; T.V. performed some of the single cell analyses, helped with preparation of movies and participated in manuscript revision; B.C.C. and M.M. designed the live animal corticosterone pulsing experiments; J.P. performed surgery, conducted the live animal timecourse experiments and performed ChIP assays; B.CC. performed the TransAM DNA binding assays and prepared figures and text for the in vivo section; M.M. performed the corticosterone EIA and qPCR; S.L. provided data for the corticosterone profile, designed experiments together with G.L.H and D.A.S., provided valuable insights into data interpretation and participated in manuscript preparation; G.L.H. oversaw the project, designed many of the experiments and, together with D.A.S., prepared most figures, coordinated work among collaborators and participated in all stages of manuscript revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1531 kb)
Supplementary Information
Supplementary Movie 1 (AVI 30125 kb)
Supplementary Information
Supplementary Movie 2 (AVI 27973 kb)
Rights and permissions
About this article
Cite this article
Stavreva, D., Wiench, M., John, S. et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 11, 1093–1102 (2009). https://doi.org/10.1038/ncb1922
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1922