Abstract
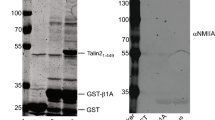
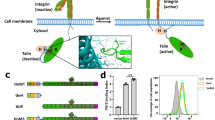
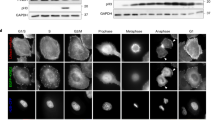
Cell migration is a dynamic process that requires temporal and spatial regulation of integrin activation and focal adhesion assembly/disassembly1. Talin, an actin and β-integrin tail-binding protein, is essential for integrin activation and focal adhesion formation2,3. Calpain-mediated cleavage of talin has a key role in focal adhesion turnover3; however, the talin head domain, one of the two cleavage products, stimulates integrin activation, localizes to focal adhesions and maintains cell edge protrusions2,4,5, suggesting that other steps, downstream of talin proteolysis, are required for focal adhesion disassembly. Here we show that talin head binds Smurf1, an E3 ubiquitin ligase involved in cell polarity and migration6,7, more tightly than full-length talin does and that this interaction leads to talin head ubiquitylation and degradation. We found that talin head is a substrate for Cdk5, a cyclin-dependent protein kinase that is essential for cell migration, synaptic transmission and cancer metastasis8,9,10,11. Cdk5 phosphorylated talin head at Ser 425, inhibiting its binding to Smurf1, thus preventing talin head ubiquitylation and degradation. Expression of the mutant talS425A, which resists Cdk5 phosphorylation thereby increasing its susceptibility to Smurf1-mediated ubiqitylation, resulted in extensive focal adhesion turnover and inhibited cell migration. Thus, talin head produced by calpain-induced cleavage of talin is degraded through Smurf1-mediated ubiquitylation; moreover, phosphorylation by Cdk5 regulates the binding of Smurf1 to talin head, controlling talin head turnover, adhesion stability and ultimately, cell migration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Webb, D. J., Parsons, J. T. & Horwitz, A. F. Adhesion assembly, disassembly and turnover in migrating cells — over and over and over again. Nature Cell Biol. 4, E97–E100 (2002).
Goksoy, E. et al. Structural basis for the autoinhibition of talin in regulating integrin activation. Mol. Cell 31, 124–133 (2008).
Franco, S. J. et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nature Cell Biol. 6, 977–983 (2004).
Nuckolls, G. H., Romer, L. H. & Burridge, K. Microinjection of antibodies against talin inhibits the spreading and migration of fibroblasts. J. Cell Sci. 102, 753–762 (1992).
Zhang, X. et al. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nature Cell Biol. 10, 1062–1068 (2008).
Wang, H.-R. et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302, 1775–1779 (2003).
Sahai, E., Garcia-Medina, R., Pouyssegur, J. & Vial, E. Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J. Cell Biol. 176, 35–42 (2007).
Cruz, J. C. & Tsai, L. H. A Jekyll and Hyde disease: roles for Cdk5 in brain development and disease. Curr. Opin. Neurobiol. 14, 390–394 (2004).
Xie, Z. G., Sanada, K., Samuels, B. A., Shih, H. & Tsai, L. H. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell 114, 469–482 (2003).
Tsai, L. H. Regulation of neuronal migration by cdk5. FASEB J. 15, A2–A2 (2001).
Strock, C. J. et al. Cyclin-dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res. 66, 7509–7515 (2006).
Nuckolls, G. H., Turner, C. E. & Burridge, K. Functional-studies of the domains of talin. J. Cell Biol. 110, 1635–1644 (1990).
Calderwood, D. A. et al. The talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 274, 28071–28074 (1999).
Di Paolo, G. et al. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 γ by the FERM domain of talin. Nature 420, 85–89 (2002).
Ling, K., Doughman, R. L., Firestone, A. J., Bunce, M. W. & Anderson, R. A. Type I γ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420, 89–93 (2002).
Chen, H. C. et al. Interaction of focal adhesion kinase with cytoskeletal protein talin. J. Biol. Chem. 270, 16995–16999 (1995).
Borowsky, M. L. & Hynes, R. O. Layilin, a novel talin-binding transmembrane protein homologous with C-type lectins, is localized in membrane ruffles. J. Cell Biol. 143, 429–442 (1998).
Wegener, K. L. et al. Structural basis for the interaction between the cytoplasmic domain of the hyaluronate receptor layilin and the talin F3 subdomain. J. Mol. Biol. 382, 112–126 (2008).
Critchley, D. R. Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion. Biochem. Soc. Trans. 32, 831–836 (2004).
Tadokoro, S. et al. Talin binding to integrin β tails: a final common step in integrin activation. Science 302, 103–106 (2003).
Petrich, B. G. et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J. Exp. Med. 204, 3103–3111 (2007).
Han, J. W. et al. Reconstructing and deconstructing agonist-induced activation of integrin α IIb β 3. Curr. Biol. 16, 1796–1806 (2006).
Priddle, H. et al. Disruption of the talin gene compromises focal adhesion assembly in undifferentiated but not differentiated embryonic stem cells. J. Cell Biol. 142, 1121–1133 (1998).
Cram, E. J., Clark, S. G. & Schwarzbauer, J. E. Talin loss-of-function uncovers roles in cell contractility and migration in C-elegans. J. Cell Sci. 116, 3871–3878 (2003).
Ratnikov, B. et al. Talin phosphorylation sites mapped by mass spectrometry. J. Cell Sci. 118, 4921–4923 (2005).
Harada, T., Morooka, T., Ogawa, S. & Nishida, E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nature Cell Biol. 3, 453–459 (2001).
Chen, F. & Studzinski, G. P. Expression of the neuronal cyclin-dependent kinase 5 activator p35Nck5a in human monocytic cells is associated with differentiation. Blood 97, 3763–3767 (2001).
Lazaro, J. et al. Cyclin dependent kinase 5, cdk5, is a positive regulator of myogenesis in mouse C2 cells. J Cell Sci. 110, 1251–1260 (1997).
Negash, S., Wang, H.-S., Gao, C., Ledee, D. & Zelenka, P. Cdk5 regulates cell-matrix and cell-cell adhesion in lens epithelial cells. J. Cell Sci. 115, 2109–2117 (2002).
Zaidel-Bar, R., Ballestrem, C., Kam, Z. & Geiger, B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116, 4605–4613 (2003).
Calderwood, D. A. et al. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 277, 21749–21758 (2002).
Ingham, R. J., Gish, G. & Pawson, T. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene 23, 1972–1984 (2004).
Huang, C., Rajfur, Z., Borchers, C., Schaller, M. D. & Jacobson, K. JNK phosphorylates paxillin and regulates cell migration. Nature 424, 219–223 (2003).
Webb, D. J. et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nature Cell Biol. 6, 154–161 (2004).
Bear, J. E. et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509–521 (2002).
Acknowledgements
We thank K. Burridge for critical reading of this manuscript, M. Schaller for critical reading of this manuscript and supporting of phosphorylation assays, A. Huttenlocher for the pEGFP–talin plasmid, L.-H. Tsai for the Cdk5 and p35 plasmids, D. Cyr for the HA-ubiquitin plasmid, E1 and Ubc5α, and M. Kerber for assistance with TIRF imaging. Supported by National Institutes of Health grants to M.H.G., a Cell Migration Consortium grant (NIH GM64346) to M.H.G. and K.J. and a Ruth L. Kirschstein National Research Service Award (1F32 HL08321) to C.H.
Author information
Authors and Affiliations
Contributions
C.H. carried out most of the experiments; Z.R. asissted with TIRF imaging, N.Y. purified talin and fragments and Z.C. made Smurf1 mutants and analysed binding to talin; M.H.G., K.J. and C.H. guided the research and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1154 kb)
Rights and permissions
About this article
Cite this article
Huang, C., Rajfur, Z., Yousefi, N. et al. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat Cell Biol 11, 624–630 (2009). https://doi.org/10.1038/ncb1868
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1868
This article is cited by
-
CDK5: an oncogene or an anti-oncogene: location location location
Molecular Cancer (2023)
-
BCKDK regulates breast cancer cell adhesion and tumor metastasis by inhibiting TRIM21 ubiquitinate talin1
Cell Death & Disease (2023)
-
Post-translational modifications of CDK5 and their biological roles in cancer
Molecular Biomedicine (2021)
-
SMURF1, a promoter of tumor cell progression?
Cancer Gene Therapy (2021)
-
Targeting the cytoskeleton against metastatic dissemination
Cancer and Metastasis Reviews (2021)