Abstract
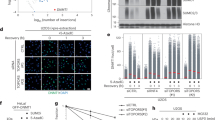
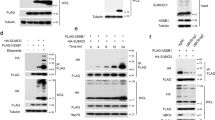
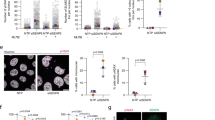
Protein modification by SUMO (small ubiquitin-like modifier) is an important regulatory mechanism for multiple cellular processes1,2. SUMO-1 modification of NEMO (NF-κB essential modulator), the IκB kinase (IKK) regulatory subunit, is critical for activation of NF-κB by genotoxic agents3. However, the SUMO ligase, and the mechanisms involved in NEMO sumoylation, remain unknown. Here, we demonstrate that although small interfering RNAs (siRNAs) against PIASy (protein inhibitor of activated STATy) inhibit NEMO sumoylation and NF-κB activation in response to genotoxic agents, overexpression of PIASy enhances these events. PIASy preferentially stimulates site-selective modification of NEMO by SUMO-1, but not SUMO-2 and SUMO–3, in vitro. PIASy–NEMO interaction is increased by genotoxic stress and occurs in the nucleus in a manner mutually exclusive with IKK interaction. In addition, hydrogen peroxide (H2O2) also increases PIASy–NEMO interaction and NEMO sumoylation, whereas antioxidants prevent these events induced by DNA-damaging agents. Our findings demonstrate that PIASy is the first SUMO ligase for NEMO whose substrate specificity seems to be controlled by IKK interaction, subcellular targeting and oxidative stress conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gill, G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 18, 2046–2059 (2004).
Hay, R. T. SUMO: a history of modification. Mol. Cell 18, 1–12 (2005).
Huang, T. T., Wuerzberger-Davis, S. M., Wu, Z. H. & Miyamoto, S. Sequential modification of NEMO/IKKγ by SUMO-1 and ubiquitin mediates NF-κB activation by genotoxic stress. Cell 115, 565–576 (2003).
Wu, Z. H., Shi, Y., Tibbetts, R. S. & Miyamoto, S. Molecular linkage between the kinase ATM and NF-κB signaling in response to genotoxic stimuli. Science 311, 1141–1146 (2006).
Pichler, A., Gast, A., Seeler, J.S., Dejean, A. & Melchior, F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 108, 109–120 (2002).
Wuerzberger-Davis, S. M., Chang, P. Y., Berchtold, C. & Miyamoto, S. Enhanced G2–M arrest by NF-κB-dependent p21waf1/cip1 induction. Mol. Cancer Res. 3, 345–353 (2005).
Bakkenist, C. J. & Kastan, M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003).
Hochstrasser, M. SP-RING for SUMO: new functions bloom for a ubiquitin-like protein. Cell 107, 5–8 (2001).
Azuma, Y., Arnaoutov, A., Anan, T. & Dasso, M. PIASy mediates SUMO-2 conjugation of Topoisomerase-II on mitotic chromosomes. EMBO J. 24, 2172–2182 (2005).
Sampson, D. A., Wang, M. & Matunis, M. J. The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J. Biol. Chem. 276, 21664–21669 (2001).
Lin, D. et al. Identification of a substrate recognition site on Ubc9. J. Biol. Chem. 277, 21740–21748 (2002).
Hayden, M. S. & Ghosh, S. Signaling to NF-κB. Genes Dev. 18, 2195–2224 (2004).
Riley, P.A. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 65, 27–33 (1994).
Pham, N.A. & Hedley, D.W. Respiratory chain-generated oxidative stress following treatment of leukemic blasts with DNA-damaging agents. Exp. Cell Res. 264, 345–352 (2001).
Mikkelsen, R.B. & Wardman, P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 22, 5734–5754 (2003).
England, K., O'Driscoll, C. & Cotter, T.G. Carbonylation of glycolytic proteins is a key response to drug-induced oxidative stress and apoptosis. Cell Death Differ. 11, 252–260 (2004).
Brummelkamp, T. R., Nijman, S. M., Dirac, A. M. & Bernards, R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424, 797–801 (2003).
Liu, B. et al. Negative regulation of NF-κB signaling by PIAS1. Mol. Cell Biol. 25, 1113–1123 (2005).
Jang, H. D., Yoon, K., Shin, Y. J., Kim, J. & Lee, S. Y. PIAS3 suppresses NF-κB-mediated transcription by interacting with the p65/RelA subunit. J. Biol. Chem. 279, 24873–24880 (2004).
Janssens, S., Tinel, A., Lippens, S. & Tschopp, J. PIDD mediates NF-κB activation in response to DNA damage. Cell 123, 1079–1092 (2005).
Karin, M. & Greten, F. R. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Rev. Immunol. 5, 749–759 (2005).
Aggarwal, B. B. Nuclear factor-κB: the enemy within. Cancer Cell 6, 203–208 (2004).
Huang, T. T. et al. NF-κB activation by camptothecin. A linkage between nuclear DNA damage and cytoplasmic signaling events. J. Biol. Chem. 275, 9501–9509 (2000).
Huang, T. T., Feinberg, S. L., Suryanarayanan, S. & Miyamoto, S. The zinc finger domain of NEMO is selectively required for NF-κB activation by UV radiation and topoisomerase inhibitors. Mol. Cell Biol. 22, 5813–5825 (2002).
Miyamoto, S., Seufzer, B. J. & Shumway, S. D. Novel IκBα proteolytic pathway in WEHI231 immature B cells. Mol. Cell Biol. 18, 19–29 (1998).
O'Connor, S., Shumway, S. D., Amanna, I. J., Hayes, C. E. & Miyamoto, S. Regulation of constitutive p50/c-Rel activity via proteasome inhibitor-resistant IκBα degradation in B cells. Mol. Cell Biol. 24, 4895–4908 (2004).
Acknowledgements
We thank Y. Azuma for generously providing recombinant purified Xenopus His–PIASy protein and Xenopus pET28a PIASy construct. We thank both M. Dasso and Y. Azuma for providing human PIASy antibody; K. Orth and S. Mukherjee for technical assistance and discussions regarding the development of in vitro sumoylation assays; E. Bresnick for the use of real time PCR equipment; P.-Y. Chang for assistance with quantitative RT–PCR analyses; S. Suryanarayanan and S. Shumway for generation of some NEMO deletion mutants. We also thank S. O'Connor for critical reading of the manuscript, C Berchtold for help with statistical analysis and the members of the Miyamoto lab for helpful discussions. This work is funded by the National Institutes of Health (NIH; T32GM008688) and Department of Defense BC044529 to A.M., Department of Defense BC010767 to S.W.-D., and NIH R01CA77474 and R01CA81065 and a Shaw Scientist Award from the Greater Milwaukee Foundation to S.M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4 and S5. (PDF 905 kb)
Rights and permissions
About this article
Cite this article
Mabb, A., Wuerzberger-Davis, S. & Miyamoto, S. PIASy mediates NEMO sumoylation and NF-κB activation in response to genotoxic stress. Nat Cell Biol 8, 986–993 (2006). https://doi.org/10.1038/ncb1458
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1458