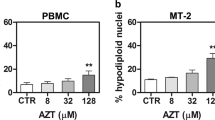
Abstract
Chemotherapy that is used to treat human immunodeficiency virus type-1 (HIV-1) infection focuses primarily on targeting virally encoded proteins. However, the combination of a short retroviral life cycle and high mutation rate leads to the selection of drug-resistant HIV-1 variants. One way to address this problem is to inhibit non-essential host cell proteins that are required for viral replication. Here we show that the activity of HIV-1 integrase stimulates an ataxia-telangiectasia-mutated (ATM)-dependent DNA damage response, and that a deficiency of this ATM kinase sensitizes cells to retrovirus-induced cell death. Consistent with these observations, we demonstrate that a novel and specific small molecule inhibitor of ATM kinase activity, KU-55933, is capable of suppressing the replication of both wild-type and drug-resistant HIV-1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nyberg, K. A., Michelson, R. J., Putnam, C. W. & Weinert, T. A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 36, 617–656 (2002).
Brown, P. O. in Retroviruses (eds Coffin, J. M., Hughes, S. H. & Varmus, H. E.) 161–203 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1997).
Daniel, R., Katz, R. A. & Skalka, A. M. A role for DNA-PK in retroviral DNA integration. Science 284, 644–647 (1999).
Li, L. et al. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J. 20, 3272–3281 (2001).
Jeanson, L. et al. Effect of Ku80 depletion on the preintegrative steps of HIV-1 replication in human cells. Virology 300, 100–108 (2002).
Kilzer, J. M. et al. Roles of host cell factors in circularization of retroviral DNA. Virology 314, 460–467 (2003).
Daniel, R. et al. Wortmannin potentiates integrase-mediated killing of lymphocytes and reduces the efficiency of stable transduction by retroviruses. Mol. Cell Biol. 21, 1164–1172 (2001).
Daniel, R. et al. Evidence that the retroviral DNA integration process triggers an ATR-dependent DNA damage response. Proc. Natl Acad. Sci. USA 100, 4778–4783 (2003).
Abraham, R. T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15, 2177–2196 (2001).
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nature Rev. Cancer 3, 155–168 (2003).
Hickson, I. et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64, 9152–9159 (2004).
Lau, A., Kanaar, R., Jackson, S. P. & O'Connor, M. J. Suppression of retroviral infection by the RAD52 DNA repair protein. EMBO J. 23, 3421–3429 (2004).
Ziv, Y. et al. Recombinant ATM protein complements the cellular AT phenotype. Oncogene 15, 159–167 (1997).
Kastan, M. B. & Lim, D. S. The many substrates and functions of ATM. Nature Rev. Mol. Cell Biol. 1, 179–186 (2000).
Shiloh, Y. ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 11, 71–77 (2001).
Leavitt, A. D., Robles, G., Alesandro, N. & Varmus, H. E. Human immunodeficiency virus type-1 integrase mutants retain in vitro integrase activity yet fail to integrate viral DNA efficiently during infection. J. Virol. 70, 721–728 (1996).
Bakkenist, C. J. & Kastan, M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003).
Zhou, B. B. et al. Caffeine abolishes the mammalian G(2)/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J. Biol. Chem. 275, 10342–10348 (2000).
Banin, S. et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281, 1674–1677 (1998).
Canman, C. E. et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281, 1677–1679 (1998).
Lim, D. S. et al. ATM phosphorylates p95/NBS1 in a S-phase checkpoint pathway. Nature 404, 613–617 (2000).
Suhnel, J. Evaluation of synergism or antagonism for the combined action of antiviral agents. Antiviral Res. 13, 23–39 (1990).
DeHart, J. L. et al. The ataxia telangiectasia-mutated and Rad3-related protein is dispensable for retroviral integration. J. Virol. 79, 1389–1396 (2005).
Ariumi, Y. et al. DNA damage sensors ATM, ATR, DNA-PKcs, and PARP-1 are dispensable for human immunodeficiency virus type-1 integration. J. Virol. 79, 2973–2978 (2005).
Sakaria, J. N. et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59, 4375–4382 (1999).
Block, W. D. et al. Selective inhibition of the DNA-dependent protein kinase (DNA-PK) by the radiosensitizing agent caffeine. Nucleic Acids Res. 32, 1967–1972 (2004).
Cortez, D. Caffeine inhibits the checkpoint responses without inhibiting the ataxia-telangiectasia-mutaed (ATM) and ATM-and-Rad3-related (ATR) protein kinases. J. Biol. Chem. 278, 37139–37145 (2003).
Foukas, L. C. et al. Direct effects of caffeine and theophylline on p110δ and other phosphoinositide 3-kinases. J. Biol. Chem. 277, 37124–37130 (2002).
Veuger, S. J. et al. Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 63, 6008–6015 (2003).
Cliby, W. A. et al. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 17, 159–169 (1998).
Tibbetts, R. S. et al. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 14, 2989–3002 (2000).
Daniel, R. et al. Histone H2AX is phosphorylated at sites of retroviral DNA integration but is dispensable for postintegration repair. J. Biol. Chem. 279, 45810–45814 (2004).
Stiff, T. et al. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 64, 2390–2396 (2004).
Gaken, J. A. et al. Efficient retroviral infection of mammalian cells is blocked by inhibition of poly(ADP-ribose) polymerase activity. J. Virol. 70, 3992–4000 (1996).
Ha, H. C. et al. Poly(ADP-ribose) polymerase-1 is required for efficient HIV-1 integration. Proc. Natl Acad. Sci. USA 98, 3364–3368 (2001).
Siva, A. C. & Bushman, F. Poly(ADP-ribose) polymerase 1 is not strictly required for infection of murine cells by retroviruses. J. Virol. 76, 11904–11910 (2002).
Rumbaugh, J. A., Fuentes, G. M. & Bambara, R. A. Processing of an HIV replication intermediate by the human DNA replication enzyme FEN1. J. Biol. Chem. 273, 28740–28745 (1998).
Faust, E. A. & Triller, H. Stimulation of human flap endonuclease 1 by human immunodeficiency virus type-1 integrase: possible role for flap endonuclease 1 in 5′-end processing of human immunodeficiency virus type-1 integration intermediates. J. Biomed. Sci. 9, 273–287 (2002).
Brown, E. J. & Baltimore, D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14, 397–402 (2000).
de Klein, A. et al. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 10, 479–482 (2000).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a novel therapeutic strategy. Nature 434, 917–921 (2005).
Borghesani, P. R. et al. Abnormal development of Purkinje cells and lymphocytes in Atm mutant mice. Proc. Natl Acad. Sci. USA 97, 3336–3341 (2000).
Roehm, N. W., Rodgers, G. H., Hatfield, S. M. & Glasebrook, A. L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immunol. Methods 142, 257–265 (1991).
Taylor, D. L. et al. Drug resistance and drug combination features of the human immunodeficiency virus inhibitor, BCH-10652 [(+/−)-2'-deoxy-3'-oxa-4'-thiocytidine, dOTC]. Antivir. Chem. Chemother. 11, 291–301 (2000).
Butler, S. L., Hansen, M. S. & Bushman, F. D. A quantitative assay for HIV DNA integration in vivo. Nature Med. 7, 631–634 (2001).
Naldini, L. et al. In vivo delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996).
Acknowledgements
We would like to acknowledge the efforts of M. Hummersone, L. Rigoreau, I. Hickson and C. Richardson for their work on KU-55933. We would also like to thank M. Albertella and A. Jazayeri for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A.l., G.C.M.S. and M.O.C. are employees of Kudos Pharmaceuticals Ltd. S.P.J. is the scientific founder and chief scientific officer of Kudos Pharmaceuticals Ltd.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3 and S4 (PDF 78 kb)
Rights and permissions
About this article
Cite this article
Lau, A., Swinbank, K., Ahmed, P. et al. Suppression of HIV-1 infection by a small molecule inhibitor of the ATM kinase. Nat Cell Biol 7, 493–500 (2005). https://doi.org/10.1038/ncb1250
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1250