Abstract
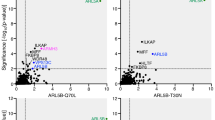
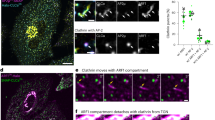
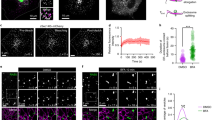
The molecular mechanisms underlying the formation of carriers trafficking from the Golgi complex to the cell surface are still ill-defined; nevertheless, the involvement of a lipid-based machinery is well established. This includes phosphatidylinositol 4-phosphate (PtdIns(4)P), the precursor for phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2). In yeast, PtdIns(4)P exerts a direct role, however, its mechanism of action and its targets in mammalian cells remain uncharacterized. We have identified two effectors of PtdIns(4)P, the four-phosphate-adaptor protein 1 and 2 (FAPP1 and FAPP2). Both proteins localize to the trans-Golgi network (TGN) on nascent carriers, and interact with PtdIns(4)P and the small GTPase ADP-ribosylation factor (ARF) through their plekstrin homology (PH) domain. Displacement or knockdown of FAPPs inhibits cargo transfer to the plasma membrane. Moreover, overexpression of FAPP-PH impairs carrier fission. Therefore, FAPPs are essential components of a PtdIns(4)P- and ARF-regulated machinery that controls generation of constitutive post-Golgi carriers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hirschberg, K. et al. Kinetic analysis of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J. Cell Biol. 143, 1485–1503 (1998).
Kreitzer, G. et al. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nature Cell Biol. 5, 126–136 (2003).
Polishchuk, R.S. et al. Correlative light-electron microscopy reveals the tubular-saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. J. Cell Biol. 148, 45–58 (2000).
Bankaitis, V.A., Malehorn, D.E., Emr, S.D. & Greene, R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J. Cell Biol. 108, 1271–1281 (1989).
Jones, S.M., Alb, J.G., Phillips, S.E. Jr, Bankaitis, V.A. & Howell, K.E. A phosphatidylinositol 3-kinase and phosphatidylinositol transfer protein act synergistically in formation of constitutive transport vesicles from the trans-Golgi network. J. Biol. Chem. 273, 10349–10354 (1998).
Simon, J.P. et al. An essential role for the phosphatidylinositol transfer protein in the scission of coatomer-coated vesicles from the trans-Golgi network. Proc. Natl Acad. Sci. USA 95, 11181–11186 (1998).
Baron, C.L. & Malhotra, V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295, 325–328 (2002).
De Matteis, M., Godi, A. & Corda, D. Phosphoinositides and the Golgi complex. Curr. Opin. Cell Biol. 14, 434–447 (2002).
Audhya, A., Foti, M. & Emr, S.D. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 11, 2673–2689 (2000).
Hama, H., Schnieders, E.A., Thorner, J., Takemoto, J.Y. & DeWald, D.B. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274, 34294–34300 (1999).
Walch-Solimena, C. & Novick, P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nature Cell Biol. 1, 523–525 (1999).
Li, X. et al. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J. Cell Biol. 157, 63–77 (2002).
Wei, Y.J. et al. Type II phosphatidylinositol 4-kinase beta is a cytosolic and peripheral membrane protein that is recruited to the plasma membrane and activated by Rac-GTP. J. Biol. Chem. 277, 46586–46593 (2002).
Nakagawa, T., Goto, K. & Kondo, H. Cloning, expression, and localization of 230-kDa phosphatidylinositol 4-kinase. J. Biol. Chem. 271, 12088–12094 (1996).
Wong, K., Meyers, R. & Cantley, L.C. Subcellular locations of phosphatidylinositol 4-kinase isoforms. J. Biol. Chem. 272, 13236–13241 (1997).
Godi, A. et al. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nature Cell Biol. 1, 280–287 (1999).
Bruns, J.R., Ellis, M.A., Jeromin, A. & Weisz, O.A. Multiple roles for phosphatidylinositol 4-kinase in biosynthetic transport in polarized Madin-Darby canine kidney cells. J. Biol. Chem. 277, 2012–2018 (2002).
Wang, Y.L. et al. Phosphotidylinositol 4 phosphate regulates tageting of clathrin adaptor AP1 complexes to the Golgi. Cell 114, 299–310 (2003).
Dowler, S. et al. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide binding specificities. Biochem. J. 351, 19–31 (2000).
Lin, X., Mattjus, P., Pike, H.M., Windebank, A.J. & Brown, R.E. Cloning and expression of glycolipid transfer protein from bovine and porcine brain. J. Biol. Chem. 275, 5104–5110 (2000).
Prescott, E.D. & Julius, D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 300, 1284–1288 (2003).
Wiedemann, C., Schafer, T. & Burger, M.M. Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J. 15, 2094–2101 (1996).
Donaldson, J.G., Finazzi, D. & Klausner, R.D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature 360, 350–352 (1992).
Helms, J.B. & Rothman, J.E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature 360, 352–354 (1992).
Levine, T.P. & Munro, S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 12, 695–704 (2002).
Stefan, C.J., Audhya, A. & Emr, S.D. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol. Biol. Cell 13, 542–557 (2002).
Cohen, G.B., Ren, R. & Baltimore, D. Modular binding domains in signal transduction proteins. Cell 80, 237–248 (1995).
Randazzo, P.A., Nie, Z., Miura, K. & Hsu, V.W. Molecular aspects of the cellular activities of ADP-ribosylation factors. Sci. STKE 59, DOI: 10.1126/stke.2000.59.re1 (2002).
Antonny, B., Huber, I., Paris, S., Chabre, M. & Cassel, D. Activation of ADP-ribosylation factor 1 GTPase-activating protein by phosphatidylcholine-derived diacylglycerols. J. Biol. Chem. 272, 30848–30851 (1997).
Jacques, K.M. et al. Arf1 dissociates from the clathrin adaptor GGA prior to being inactivated by Arf GTPase-activating proteins. J. Biol. Chem. 277, 47235–47241 (2002).
Puertollano, R., Randazzo, P.A., Presley, J.F., Hartnell, L.M. & Bonifacino, J.S. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell 105, 93–102 (2001).
Yao, L., Kawakami, Y. & Kawakami, T. The pleckstrin homology domain of Bruton tyrosine kinase interacts with protein kinase C. Proc. Natl Acad. Sci. USA 91, 9175–9179 (1994).
Touhara, K., Inglese, J., Pitcher, J.A., Shaw, G. & Lefkowitz, R.J. Binding of G protein beta gamma-subunits to pleckstrin homology domains. J. Biol. Chem. 269, 10217–10220 (1994).
Snyder, J.T., Singer, A.U., Wing, M.R., Harden, T.K. & Sondek, J. The pleckstrin homology domain of phospholipase C-beta2 as an effector site for Rac. J. Biol. Chem. 278, 21099–21104 (2003).
Rossman, K.L. et al. A crystallographic view of interactions between Dbs and Cdc42: PH domain-assisted guanine nucleotide exchange. EMBO J. 21, 1315–1326 (2002).
Burger, K.N. Greasing membrane fusion and fission machineries. Traffic 1, 605–613 (2000).
Corda, D., Hidalgo-Carcedo, C., Bonazzi, M., Luini, A. & Spano, S. Molecular aspects of membrane fission in the secretory pathway. Cell. Mol. Life Sci. 59, 1819–1832 (2002).
Ikonen, E. & Simons, K. Protein and lipid sorting from the trans-Golgi network to the plasma membrane in polarized cells. Semin. Cell Dev. Biol. 9, 503–509 (1998).
Raya, A. et al. Goodpasture antigen-binding protein, the kinase that aphosphorylates the goodpasture antigen, is an alternatively spliced variant implicated in autoimmune pathogenesis. J. Biol. Chem. 275, 40392–40399 (2000).
Soccio, R.E. & Breslow, J.L. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J. Biol. Chem. 278, 22183–22186 (2003).
Hanada, K. et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature 426, 803–809 (2003).
Marra, P. et al. The GM130 and GRASP65 Golgi proteins cycle through and define a subdomain of the intermediate compartment. Nature Cell Biol. 3, 1101–1113 (2001).
Godi, A. et al. ADP ribosylation factor regulates spectrin binding to the Golgi complex. Proc. Natl Acad. Sci. USA 95, 8607–8612 (1998).
Randazzo, P.A. & Kahn, R.A. Myristoylation and ADP-ribosylation factor function. Methods Enzymol. 250, 394–405 (1995).
Buccione, R. et al. Regulation of constitutive exocytic transport by membrane receptors. A biochemical and morphometric study. J. Biol. Chem. 271, 3523–3533 (1996).
Lucocq, J.M. in Fine Structure Immunocytochemistry (ed. Griffiths, G.) 279–302 (Springer, Berlin, 1993).
Acknowledgements
We thank B. Antonny for providing reagents. We are also grateful to A. Luini, V. Malhotra, and to the members of the Golgi group of the Consorzio Mario Negri Sud for discussions. We thank C. Berrie for reading the manuscript and E. Fontana for artwork preparation. This work was supported in part by the Italian Association for Cancer Research, Telethon Italia, the European Community and the Italian Ministry of Education. A.D.C. and T.D. are supported by fellowships from the Italian Foundation of Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Godi, A., Campli, A., Konstantakopoulos, A. et al. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol 6, 393–404 (2004). https://doi.org/10.1038/ncb1119
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1119