Abstract
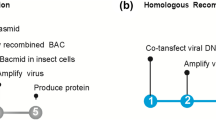
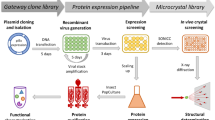
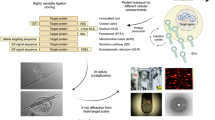
The discovery of large multiprotein complexes in cells has increased the demand for improved heterologous protein production techniques to study their molecular structure and function. Here we describe MultiBac, a simple and versatile system for generating recombinant baculovirus DNA to express protein complexes comprising many subunits. Our method uses transfer vectors containing a multiplication module that can be nested to facilitate assembly of polycistronic expression cassettes, thereby minimizing requirements for unique restriction sites. The transfer vectors access a modified baculovirus DNA through Cre-loxP site-specific recombination or Tn7 transposition. This baculovirus has improved protein expression characteristics because specific viral genes have been eliminated. Gene insertion reactions are carried out in Escherichia coli either sequentially or concurrently in a rapid, one-step procedure. Our system is useful for both recombinant multiprotein production and multigene transfer applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Uetz, P. et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae . Nature 403, 623–627 (2000).
Giot, L. et al. A protein interaction map of Drosophila melanogaster . Science 302, 1727–1736 (2003).
Rigaut, G. et al. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17, 1030–1032 (1999).
Forler, D. et al. An efficient protein complex purification method for functional proteomics in higher eukaryotes. Nat. Biotechnol. 21, 89–92 (2003).
Gavin, A.C. et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147 (2002).
Ho, Y. et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415, 180–183 (2002).
Schwikowski, B., Uetz, P. & Fields, S. A network of protein-protein interactions in yeast. Nat. Biotechnol. 18, 1257–1261 (2000).
von Mering, C. et al. Comparative assessment of large-scale data sets of protein-protein interactions. Nature 417, 399–403 (2002).
Grigoriev, A. On the number of protein-protein interactions in the yeast proteome. Nucleic Acids Res. 31, 4157–4161 (2003).
Alberts, B. The cell as a collection of protein machines: preparing the next generation of molecular biologists. Cell 92, 291–294 (1998).
Selleck, W. et al. A histone fold TAF octamer within the yeast TFIID transcriptional coactivator. Nat. Struct. Biol. 8, 695–700 (2001).
de Felipe, P. Polycistronic viral vectors. Curr. Gene Ther. 2, 355–378 (2002).
Planelles, V. Hybrid lentivirus vectors. Methods Mol. Biol. 229, 273–284 (2003).
O'Reilly, D.R., Miller, L.K. & Luckow, V.A. Baculovirus expression vectors. A laboratory manual. (Oxford University Press, New York, 1994).
Roy, P., Mikhailov, M. & Bishop, D.H. Baculovirus multigene expression vectors and their use for understanding the assembly process of architecturally complex virus particles. Gene 190, 119–129 (1997).
Bertolotti-Ciarlet, A., Ciarlet, M., Crawford, S.E., Conner, M.E. & Estes, M.K. Immunogenicity and protective efficacy of rotavirus 2/6-virus-like particles produced by a dual baculovirus expression vector and administered intramuscularly, intranasally, or orally to mice. Vaccine 21, 3885–3900 (2003).
Luckow, V.A., Lee, S.C., Barry, G.F. & Olins, P.O. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli . J. Virol. 67, 4566–4579 (1993).
Bac-to-Bac Baculoviorus Expression Systems Manual. (Invitrogen, Life Technologies Inc., 2000).
Liu, Q., Li, M.Z., Leibham, D., Cortez, D. & Elledge, S.J. The univector plasmid-fusion system, a method for rapid construction of recombinant DNA without restriction enzymes. Curr. Biol. 8, 1300–1309 (1998).
Metcalf, W.W., Jiang, W. & Wanner, B.L. Use of the rep technique for allele replacement to construct new Escherichia coli hosts for maintenance of R6K gamma origin plasmids at different copy numbers. Gene 138, 1–7 (1994).
Slack, J.M., Kuzio, J. & Faulkner, P. Characterization of v-cath, a cathepsin L-like proteinase expressed by the baculovirus Autographa californica multiple nuclear polyhedrosis virus. J. Gen. Virol. 76, 1091–1098 (1995).
Suzuki, T. et al. Efficient protein production using a Bombyx mori nuclear polyhedrosis virus lacking the cysteine proteinase gene. J. Gen. Virol. 78, 3073–3080 (1997).
Hom, L.G. & Volkman, L.E. Autographa californica M nucleopolyhedrovirus chiA is required for processing of V-CATH. Virology 277, 178–183 (2000).
Fitzgerald, D.J. et al. Reaction cycle of the yeast Isw2 chromatin remodeling complex. EMBO J. 23, 3836–3843 (2004).
Patterson, G., Day, R.N. & Piston, D. Fluorescent protein spectra. J. Cell. Sci. 114, 837–838 (2001).
Kost, T.A. & Condreay, J.P. Recombinant baculoviruses as mammalian cell gene-delivery vectors. Trends Biotechnol. 20, 173–180 (2002).
Huser, A., Rudolph, M. & Hofmann, C. Incorporation of decay-accelerating factor into the baculovirus envelope generates complement-resistant gene transfer vectors. Nat. Biotechnol. 19, 451–455 (2001).
Zhang, Y., Buchholz, F., Muyrers, J.P. & Stewart, A.F. A new logic for DNA engineering using recombination in Escherichia coli . Nat. Genet. 20, 123–128 (1998).
Guzman, L.M., Belin, D., Carson, M.J. & Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130 (1995).
Shizuya, H. et al. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc. Natl. Acad. Sci. USA 89, 8794–8797 (1992).
Acknowledgements
We thank Christiane Schaffitzel and Ralf Wellinger for discussions, as well as Philipp Berger, Yvonne Hunziker, Martina Mijuskovic, Carl DeLuca and Robert Coleman for assistance. We appreciate helpful comments on baculovirus expression from Robert Noad and Polly Roy. I.B. was a Liebig fellow of the Fonds der Chemischen Industrie (FCI, Germany), D.J.F. was supported by a Human Frontiers Science Program (HSFP) post-doctoral fellowship. We appreciate support from the Swiss National Fund through membership in the National Center for Competence in Research Structural Biology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A provisional US patent application no 60/552.072 has been filed.
Supplementary information
Supplementary Fig. 1
Replacement of chiA and v-cath by LoxP. (PDF 136 kb)
Rights and permissions
About this article
Cite this article
Berger, I., Fitzgerald, D. & Richmond, T. Baculovirus expression system for heterologous multiprotein complexes. Nat Biotechnol 22, 1583–1587 (2004). https://doi.org/10.1038/nbt1036
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1036
This article is cited by
-
A sensitive assay for scrutiny of Autographa californica multiple nucleopolyhedrovirus genes using CRISPR-Cas9
Applied Microbiology and Biotechnology (2023)
-
HR-Bac, a toolbox based on homologous recombination for expression, screening and production of multiprotein complexes using the baculovirus expression system
Scientific Reports (2022)
-
Enhancer RNAs stimulate Pol II pause release by harnessing multivalent interactions to NELF
Nature Communications (2022)
-
Structure of an MHC I–tapasin–ERp57 editing complex defines chaperone promiscuity
Nature Communications (2022)
-
Structural Organization of Human Full-Length PAR3 and the aPKC–PAR6 Complex
Molecular Biotechnology (2022)