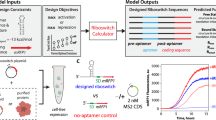
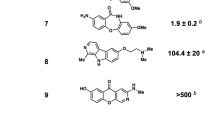
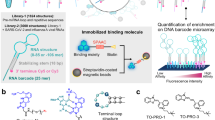
Abstract
Most approaches to monitoring interactions between biological macromolecules require large amounts of material, rely upon the covalent modification of an interaction partner, or are not amenable to real-time detection. We have developed a generalizable assay system based on interactions between proteins and reporter ribozymes. The assay can be configured in a modular fashion to monitor the presence and concentration of a protein or of molecules that modulate protein function. We report two applications of the assay: screening for a small molecule that disrupts protein binding to its nucleic acid target and screening for protein–protein interactions. We screened a structurally diverse library of antibiotics for small molecules that modulate the activity of HIV-1 Rev-responsive ribozymes by binding to Rev. We identified an inhibitor that subsequently inhibited HIV-1 replication in cells. A simple format switch allowed reliable monitoring of domain-specific interactions between the blood-clotting factor thrombin and its protein partners. The rapid identification of interactions between proteins or of compounds that disrupt such interactions should have substantial utility for the drug-discovery process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thompson, L. & Ellman, J. Synthesis and applications of small molecule libraries. Chem. Rev. 96, 555–600 (1996).
Jayawickreme, C.K. & Kost, T.A. Gene expression systems in the development of high-throughput screens. Curr. Opin. Biotechnol. 8, 629–634 (1997).
Rademann, J. & Jung, G. Drug discovery. Integrating combinatorial synthesis and bioassays. Science 287, 1947–1948 (1998).
Mendelsohn, A.R. & Brent, R. Protein interaction methods—toward an endgame. Science 284, 1948–1950 (1999).
Pandey, A. & Mann, M. Proteomics to study genes and genomes. Nature 405, 837–846 (2000).
Phizicky, E.M. & Fields, S. Protein–protein interactions: methods for detection and analysis. Microbiol. Rev. 59, 94–123 (1995).
Cech, T.R. in The RNA World (eds Gesteland, R.F. & Atkins, J.F.) 239–269 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1993).
Warashina, M., Takagi, Y., Stec, W.J. & Taira, K. Differences among mechanisms of ribozyme-catalyzed reactions. Curr. Opin. Biotechnol. 11, 354–362 (2000).
Long, D.M. & Uhlenbeck, O.C. Self-cleaving catalytic RNA. FASEB J. 7, 25–30 (1993).
Doherty, E.A. & Doudna, J.A. Ribozyme structures and mechanisms. Annu. Rev. Biochem. 69, 597–615 (2000).
Walter, N.G. & Burke, J.M. The hairpin ribozyme: structure, assembly and catalysis. Curr. Opin. Chem. Biol. 2, 24–30 (1998).
Verma, S., Vaish, N.K. & Eckstein, F. Structure–function studies of the hammerhead ribozyme. Curr. Opin. Chem. Biol. 1, 532–536 (1997).
Rossi, J.J. Ribozymes, genomics and therapeutics. Chem. Biol. 6, R33–R37 (1999).
Bramlage, B., Luzi, E. & Eckstein, F. Designing ribozymes for the inhibition of gene expression. Trends Biotechnol. 16, 434–438 (1998).
Sullenger, B.A. & Cech, T.R. Ribozyme-mediated repair of defective mRNA by targeted, trans-splicing. Nature 371, 619–622 (1994).
Sullenger, B.A. Revising messages traveling along the cellular information superhighway. Chem. Biol. 2, 249–253 (1995).
Jenne, A., Gmelin, W., Raffler, N. & Famulok, M. Real-time characterization of ribozymes by fluorescence resonance energy transfer (FRET). Angew. Chem. Int. Ed. 38, 1300–1303 (1999).
Jenne, A. et al. Rapid identification and characterization of hammerhead-ribozyme inhibitors using fluorescence-based technology. Nat. Biotechnol. 19, 56–61 (2001).
Soukup, G.A. & Breaker, R.R. Engineering precision RNA molecular switches. Proc. Natl. Acad. Sci. USA 96, 3584–3589 (1999).
Peterson, R.D. & Feigon, J. Structural change in Rev responsive element RNA of HIV-1 on binding Rev peptide. J. Mol. Biol. 264, 863–877 (1996).
Gosser, Y. et al. Peptide-triggered conformational switch in HIV-1 RRE RNA complexes. Nat. Struct. Biol. 8, 146–150 (2001).
Giver, L. et al. Selective optimization of the Rev-binding element of HIV-1. Nucleic Acids Res. 21, 5509–5516 (1993).
Battiste, J.L. et al. Alpha helix-RNA major groove recognition in an HIV-1 rev peptide-RRE RNA complex. Science 273, 1547–1551 (1996).
Hung, L.W., Holbrook, E.L. & Holbrook, S.R. The crystal structure of the Rev binding element of HIV-1 reveals novel base pairing and conformational variability. Proc. Natl. Acad. Sci. USA 97, 5107–5112 (2000).
Ippolito, J.A. & Steitz, T.A. The structure of the HIV-1 RRE high affinity rev binding site at 1.6 Å resolution. J. Mol. Biol. 295, 711–717 (2000).
Bohan, C.A. et al. Analysis of Tat transactivation of human immunodeficiency virus transcription in vitro. Gene Expr. 2, 391–407 (1992).
Burd, C.G. & Dreyfuss, G. Conserved structures and diversity of functions of RNA-binding proteins. Science 265, 615–621 (1994).
Mattaj, I.W. RNA recognition: a family matter? Cell 73, 837–840 (1993).
Patel, D.J. Adaptive recognition in RNA complexes with peptides and protein modules. Curr. Opin. Struct. Biol. 9, 74–87 (1999).
Esteban, J.A., Banerjee, A.R. & Burke, J.M. Kinetic mechanism of the hairpin ribozyme. Identification and characterization of two nonexchangeable conformations. J. Biol. Chem. 272, 13629–13639 (1997).
Bock, L.C., Griffin, L.C., Latham, J.A., Vermaas, E.H. & Toole, J.J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 355, 564–566 (1992).
Rezaie, A.R. Heparin-binding exosite of factor Xa. Trends Cardiovasc. Med. 10, 333–338 (2000).
Rydel, T.J. et al. The structure of a complex of recombinant hirudin and human α-thrombin. Science 249, 277–280 (1990).
Niehrs, C., Huttner, W.B., Carvallo, D. & Degryse, E. Conversion of recombinant hirudin to the natural form by in vitro tyrosine sulfation. Differential substrate specificities of leech and bovine tyrosylprotein sulfotransferases. J. Biol. Chem. 265, 9314–9318 (1990).
Famulok, M., Blind, M. & Mayer, G. Intramers as promising new tools in functional proteomics. Chem. Biol. 8, 931–939 (2001).
Cox, J.C. & Ellington, A.D. Automated selection of anti-protein aptamers. Bioorg. Med. Chem. 9, 2525–2531 (2001).
Herschlag, D., Khosla, M., Tsuchihashi, Z. & Karpel, R.L. An RNA chaperone activity of non-specific RNA binding proteins in hammerhead ribozyme catalysis. EMBO J. 13, 2913–2924 (1994).
Robertson, M.P. & Ellington, A.D. In vitro selection of nucleoprotein enzymes. Nat. Biotechnol. 19, 650–655 (2001).
Atsumi, S., Ikawa, Y., Shiraishi, H. & Inoue, T. Design and development of a catalytic ribonucleoprotein. EMBO J. 20, 5453–5460 (2001).
Nissen, P., Hansen, J., Ban, N., Moore, P.B. & Steitz, T.A. The structural basis of ribosome activity in peptide bond synthesis. Science 289, 920–930 (2000).
Valadkhan, S. & Manley, J.L. Splicing-related catalysis by protein-free snRNAs. Nature 413, 701–707 (2001).
Herschlag, D. RNA chaperones and the RNA folding problem. J. Biol. Chem. 270, 20871–20874 (1995).
Robertson, M.P. & Ellington, A.D. Design and optimization of effector-activated ribozyme ligases. Nucleic Acids Res. 28, 1751–1759 (2000).
Jose, A.M., Soukup, G.A. & Breaker, R.R. Cooperative binding of effectors by an allosteric ribozyme. Nucleic Acids Res. 29, 1631–1637 (2001).
Vitiello, D., Pecchia, D.B. & Burke, J.M. Intracellular ribozyme-catalyzed trans-cleavage of RNA monitored by fluorescence resonance energy transfer. RNA 6, 628–637 (2000).
Geiger, C. et al. Cytohesin-1 regulates β-2 integrin-mediated adhesion through both ARF-GEF function and interaction with LFA-1. EMBO J. 19, 2525–2536 (2000).
Mayer, G. et al. Controlling small guanine-nucleotide-exchange factor function through cytoplasmic RNA intramers. Proc. Natl. Acad. Sci. USA 98, 4961–4965 (2001).
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (to M.F.) and by National Institutes of Health grants AI-36083 and GM-61789 (to A.D.E.). We thank Julian Davies (University of Vancouver) for providing the antibiotics library, Christoph Müller (European Molecular Biology Laboratory, Grenoble) for NFκB, p52, and Bcl-3, Tobias Restle (Max-Planck-Insititut für Molekulare Physiologie, Dortmund) for HIV-RT, Günter Mayer for helpful discussions, and Michael Hoch and Waldemar Kolanus (University of Bonn) for comments on the manuscript. The following reagents were obtained through the National Institutes of Health AIDS Research and Reference Reagent Program (National Institute of Allergy and Infectious Diseases): HIV-1 Tat protein from John Brady and H9 cells and HIV-1IIIb from Robert Gallo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A patent application on this work has been submitted on behalf of Nascacell GmbH, (Tutzing, Germany).
Rights and permissions
About this article
Cite this article
Hartig, J., Najafi-Shoushtari, S., Grüne, I. et al. Protein-dependent ribozymes report molecular interactions in real time. Nat Biotechnol 20, 717–722 (2002). https://doi.org/10.1038/nbt0702-717
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0702-717
This article is cited by
-
A general design strategy for protein-responsive riboswitches in mammalian cells
Nature Methods (2014)
-
Generation and selection of ribozyme variants with potential application in protein engineering and synthetic biology
Applied Microbiology and Biotechnology (2014)
-
Aptamers for allosteric regulation
Nature Chemical Biology (2011)
-
Autocatalytic aptazymes enable ligand-dependent exponential amplification of RNA
Nature Biotechnology (2009)