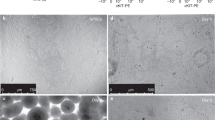
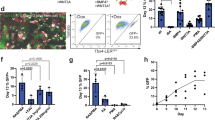
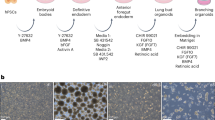
Abstract
The ability to generate lung and airway epithelial cells from human pluripotent stem cells (hPSCs) would have applications in regenerative medicine, modeling of lung disease, drug screening and studies of human lung development. We have established, based on developmental paradigms, a highly efficient method for directed differentiation of hPSCs into lung and airway epithelial cells. Long-term differentiation of hPSCs in vivo and in vitro yielded basal, goblet, Clara, ciliated, type I and type II alveolar epithelial cells. The type II alveolar epithelial cells were capable of surfactant protein-B uptake and stimulated surfactant release, providing evidence of specific function. Inhibiting or removing retinoic acid, Wnt and BMP—agonists to signaling pathways critical for early lung development in the mouse—recapitulated defects in corresponding genetic mouse knockouts. As this protocol generates most cell types of the respiratory system, it may be useful for deriving patient-specific therapeutic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Green, M.D., Huang, S.X. & Snoeck, H.W. Stem cells of the respiratory system: from identification to differentiation into functional epithelium. Bioessays 35, 261–270 (2013).
Rock, J.R. & Hogan, B.L. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu. Rev. Cell Dev. Biol. 27, 493–512 (2011).
Morrisey, E.E. & Hogan, B.L. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell 18, 8–23 (2010).
Rawlins, E.L., Clark, C.P., Xue, Y. & Hogan, B.L. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136, 3741–3745 (2009).
Green, M.D. et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 29, 267–272 (2011).
Mou, H. et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 10, 385–397 (2012).
Longmire, T.A. et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10, 398–411 (2012).
Wong, A.P. et al. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTRTR protein. Nat. Biotechnol. 30, 876–882 (2012).
Kubo, A. et al. Development of definitive endoderm from embryonic stem cells in culture. Development 131, 1651–1662 (2004).
Nostro, M.C. & Keller, G. Generation of beta cells from human pluripotent stem cells: potential for regenerative medicine. Semin. Cell Dev. Biol. 23, 701–710 (2012).
Nostro, M.C. et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 138, 861–871 (2011).
D'Amour, K.A. et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 (2005).
Goss, A.M. et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell 17, 290–298 (2009).
Bellusci, S., Grindley, J., Emoto, H., Itoh, N. & Hogan, B.L. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124, 4867–4878 (1997).
Bellusci, S., Henderson, R., Winnier, G., Oikawa, T. & Hogan, B.L. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 122, 1693–1702 (1996).
Domyan, E.T. et al. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development 138, 971–981 (2011).
Li, Y., Gordon, J., Manley, N.R., Litingtung, Y. & Chiang, C. Bmp4 is required for tracheal formation: a novel mouse model for tracheal agenesis. Dev. Biol. 322, 145–155 (2008).
Chen, F. et al. A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J. Clin. Invest. 120, 2040–2048 (2010).
Yamamoto, M. et al. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature 428, 387–392 (2004).
Perea-Gomez, A. et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev. Cell 3, 745–756 (2002).
del Barco Barrantes, I., Davidson, G., Grone, H.J., Westphal, H. & Niehrs, C. Dkk1 and noggin cooperate in mammalian head induction. Genes Dev. 17, 2239–2244 (2003).
Yu, P.B. et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 4, 33–41 (2008).
Inman, G.J. et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62, 65–74 (2002).
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 (2009).
Bennett, C.N. et al. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 277, 30998–31004 (2002).
Fusaki, N., Ban, H., Nishiyama, A., Saeki, K. & Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 85, 348–362 (2009).
Warren, L. et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 (2010).
Kimura, S. et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 10, 60–69 (1996).
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480, 547–551 (2011).
Harris-Johnson, K.S., Domyan, E.T., Vezina, C.M. & Sun, X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc. Natl. Acad. Sci. USA 106, 16287–16292 (2009).
Rock, J.R. et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 106, 12771–12775 (2009).
Weaver, M., Yingling, J.M., Dunn, N.R., Bellusci, S. & Hogan, B.L. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development 126, 4005–4015 (1999).
Shu, W. et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev. Biol. 283, 226–239 (2005).
Post, M. et al. Keratinocyte growth factor and its receptor are involved in regulating early lung branching. Development 122, 3107–3115 (1996).
Malpel, S., Mendelsohn, C. & Cardoso, W.V. Regulation of retinoic acid signaling during lung morphogenesis. Development 127, 3057–3067 (2000).
Wongtrakool, C. et al. Down-regulation of retinoic acid receptor alpha signaling is required for sacculation and type I cell formation in the developing lung. J. Biol. Chem. 278, 46911–46918 (2003).
Gonzales, L.W., Guttentag, S.H., Wade, K.C., Postle, A.D. & Ballard, P.L. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am. J. Physiol. Lung Cell. Mol. Physiol. 283, L940–L951 (2002).
Sanchez-Esteban, J. et al. Mechanical stretch promotes alveolar epithelial type II cell differentiation. J. Appl. Physiol. 91, 589–595 (2001).
Whitsett, J.A., Wert, S.E. & Weaver, T.E. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu. Rev. Med. 61, 105–119 (2010).
Rooney, S.A. Regulation of surfactant secretion. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129, 233–243 (2001).
Gouon-Evans, V. et al. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat. Biotechnol. 24, 1402–1411 (2006).
Osafune, K. et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol. 26, 313–315 (2008).
Bock, C. et al. Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144, 439–452 (2011).
Boulting, G.L. et al. A functionally characterized test set of human induced pluripotent stem cells. Nat. Biotechnol. 29, 279–286 (2011).
Blauwkamp, T.A., Nigam, S., Ardehali, R., Weissman, I.L. & Nusse, R. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nat. Commun. 3, 1070 (2012).
Barkauskas, C.E. et al. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 123, 3025–3036 (2013).
Xu, X. et al. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc. Natl. Acad. Sci. USA 109, 4910–4915 (2012).
O'Neill, J.D. et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann. Thorac. Surg. 96, 1046–1056 (2013).
Acknowledgements
S.X.L.H. is a Druckenmiller Fellow of the New York Stem Cell Foundation. The authors wish to thank J. Sonnet and the Columbia Lung Regeneration Team, D. Farber and J. Thome for providing samples of human lung tissue; K. Brown for kind help with electron microscopy; S.-H. Ho for assistance with microscopy.
Author information
Authors and Affiliations
Contributions
S.X.L.H. performed most experiments, developed this protocol and co-wrote the manuscript; M.N.I. performed functional analysis of ATII cells supervised by J.B.; M.M., M.D.G. and Y.-W.C. gave technical advice and assisted S.X.L.H. experimentally; Z.H. performed kidney capsule transplantations supervised by Y.-G.Y.; J.O.N. provided human lung human decellularized extracellular lung matrix and these experiments were supervised by G.V.-N.; H.-W.S. developed the concept, co-analyzed primary data and co-wrote the manuscript with S.X.L.H.
Corresponding author
Ethics declarations
Competing interests
The authors have filed patent application IRCU13340.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Tables 1 and 2 (PDF 2840 kb)
Rights and permissions
About this article
Cite this article
Huang, S., Islam, M., O'Neill, J. et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32, 84–91 (2014). https://doi.org/10.1038/nbt.2754
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2754