Abstract
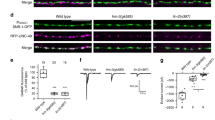
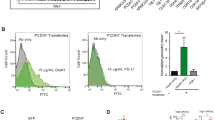
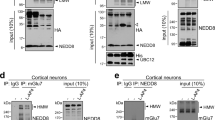
In cells, molecular motors operate in polarized sorting of molecules, although the steering mechanisms of motors remain elusive1. In neurons, the kinesin motor2 conducts vesicular transport such as the transport of synaptic vesicle components to axons3 and of neurotransmitter receptors to dendrites4, indicating that vesicles may have to drive the motor for the direction to be correct. Here we show that an AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionate) receptor subunit—GluR2-interacting protein (GRIP1)—can directly interact and steer kinesin heavy chains to dendrites as a motor for AMPA receptors. As would be expected if this complex is functional, both gene targeting and dominant negative experiments of heavy chains of mouse kinesin showed abnormal localization of GRIP1. Moreover, expression of the kinesin-binding domain of GRIP1 resulted in accumulation of the endogenous kinesin predominantly in the somatodendritic area. This pattern was different from that generated by the overexpression of the kinesin-binding scaffold protein JSAP1 (JNK/SAPK-associated protein-1, also known as Mapk8ip3), which occurred predominantly in the somatoaxon area. These results indicate that directly binding proteins can determine the traffic direction of a motor protein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hirokawa, N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279, 519–526 (1998)
Vale, R. D., Reese, T. S. & Sheetz, M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42, 39–50 (1985)
Ferreira, A., Niclas, J., Vale, R. D., Banker, G. & Kosik, K. S. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J. Cell Biol. 117, 595–606 (1992)
Kim, C. H. & Lisman, J. E. A labile component of AMPA receptor-mediated synaptic transmission is dependent on microtubule motors, actin, and N-ethylmaleimide-sensitive factor. J. Neurosci. 21, 4188–4194 (2001)
Burack, M. A., Silverman, M. A. & Banker, G. The role of selective transport in neuronal protein sorting. Neuron 26, 465–472 (2000)
Severt, W. L. et al. The suppression of testis-brain RNA binding protein and kinesin heavy chain disrupts mRNA sorting in dendrites. J. Cell Sci. 112, 3691–3702 (1999)
Setou, M., Nakagawa, T., Seog, D. H. & Hirokawa, N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 288, 1796–1802 (2000)
Dong, H. et al. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386, 279–284 (1997)
Wyszynski, M. et al. Association of AMPA receptors with a subset of glutamate receptor-interacting protein in vivo. J. Neurosci. 19, 6528–6537 (1999)
Skoufias, D. A., Cole, D. G., Wedaman, K. P. & Scholey, J. M. The carboxyl-terminal domain of kinesin heavy chain is important for membrane binding. J. Biol. Chem. 269, 1477–1485 (1994)
Seiler, S. et al. Cargo binding and regulatory sites in the tail of fungal conventional kinesin. Nature Cell Biol. 2, 333–338 (2000)
Tanaka, Y. et al. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell 93, 1147–1158 (1998)
Bruckner, K. et al. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 22, 511–524 (1999)
Kanai, Y. et al. KIF5C, a novel neuronal kinesin enriched in motor neurons. J. Neurosci. 20, 6374–6384 (2000)
Rahman, A., Kamal, A., Roberts, E. A. & Goldstein, L. S. Defective kinesin heavy chain behaviour in mouse kinesin light chain mutants. J. Cell Biol. 146, 1277–1288 (1999)
Rubio, M. E. & Wenthold, R. J. Differential distribution of intracellular glutamate receptors in dendrites. J. Neurosci. 19, 5549–5562 (1999)
Verhey, K. J. et al. Cargo of kinesin identified as JIP scaffolding proteins and associated signalling molecules. J. Cell Biol. 152, 959–970 (2001)
Lee, S. H., Valtschanoff, J. G., Kharazia, V. N., Weinberg, R. & Sheng, M. Biochemical and morphological characterization of an intracellular membrane compartment containing AMPA receptors. Neuropharmacology 41, 680–692 (2001)
Ye, B. et al. GRASP-1: a neuronal RasGEF associated with the AMPA receptor/GRIP complex. Neuron 26, 603–617 (2000)
Ito, M. et al. JSAP1, a novel jun N-terminal protein kinase (JNK)-binding protein that functions as a Scaffold factor in the JNK signalling pathway. Mol. Cell. Biol. 19, 7539–7548 (1999)
Bowman, A. B. et al. Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell 103, 583–594 (2000)
Baas, P. W., Deitch, J. S., Black, M. M. & Banker, G. A. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl Acad. Sci. USA 85, 8335–8339 (1988)
Niclas, J., Navone, F., Hom-Booher, N. & Vale, R. D. Cloning and localization of a conventional kinesin motor expressed exclusively in neurons. Neuron 12, 1059–1072 (1994)
Marszalek, J. R., Weiner, J. A., Farlow, S. J., Chun, J. & Goldstein, L. S. Novel dendritic kinesin sorting identified by different process targeting of two related kinesins: KIF21A and KIF21B. J. Cell Biol. 145, 469–479 (1999)
Toyoshima, I., Yu, H., Steuer, E. R. & Sheetz, M. P. Kinectin, a major kinesin-binding protein on ER. J. Cell Biol. 118, 1121–1131 (1992)
Huang, J. D. et al. Direct interaction of microtubule- and actin-based transport motors. Nature 397, 267–270 (1999)
Kamal, A., Stokin, G. B., Yang, Z., Xia, C. H. & Goldstein, L. S. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron 28, 449–459 (2001)
Burette, A. et al. Differential cellular and subcellular localization of AMPA receptor-binding protein and glutamate receptor-interacting protein. J. Neurosci. 21, 495–503 (2001)
Brewer, G. J. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J. Neurosci. Res. 42, 674–683 (1995)
Acknowledgements
We thank R. L. Huganir for the anti-GRIP1 and anti-GRIP2 antibodies, M. Mishina for anti-NR2B antibody, and M. Sheng for an anti-PanGRIP antibody. We thank Y. Hayashi for advice and for the Sindbis virus construction. We also thank Y. Hata, T. Nakagawa and M. Sheng for discussions; and H. Sato, H. Fukuda, T. Matsuki and M. Sugaya for technical assistance. Finally, we thank other members of the Hirokawa laboratory, especially T. Nakata, for technical assistance, discussions, and advice throughout the conducting of experiments. This work is supported by a Center of Excellence Grant-in-Aid from the Ministry of Education, Science, Sports, Culture and Technology of Japan to N. Hirokawa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests
Rights and permissions
About this article
Cite this article
Setou, M., Seog, DH., Tanaka, Y. et al. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature 417, 83–87 (2002). https://doi.org/10.1038/nature743
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature743