Abstract
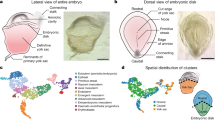
In mammals, specification of the three major germ layers occurs during gastrulation, when cells ingressing through the primitive streak differentiate into the precursor cells of major organ systems. However, the molecular mechanisms underlying this process remain unclear, as numbers of gastrulating cells are very limited. In the mouse embryo at embryonic day 6.5, cells located at the junction between the extra-embryonic region and the epiblast on the posterior side of the embryo undergo an epithelial-to-mesenchymal transition and ingress through the primitive streak. Subsequently, cells migrate, either surrounding the prospective ectoderm contributing to the embryo proper, or into the extra-embryonic region to form the yolk sac, umbilical cord and placenta. Fate mapping has shown that mature tissues such as blood and heart originate from specific regions of the pre-gastrula epiblast1, but the plasticity of cells within the embryo and the function of key cell-type-specific transcription factors remain unclear. Here we analyse 1,205 cells from the epiblast and nascent Flk1+ mesoderm of gastrulating mouse embryos using single-cell RNA sequencing, representing the first transcriptome-wide in vivo view of early mesoderm formation during mammalian gastrulation. Additionally, using knockout mice, we study the function of Tal1, a key haematopoietic transcription factor, and demonstrate, contrary to previous studies performed using retrospective assays2,3, that Tal1 knockout does not immediately bias precursor cells towards a cardiac fate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Data deposits
ChIP-seq data are available at the NCBI Gene Expression Omnibus portal under accession number GSE74994. Processed data are also available at http://codex.stemcells.cam.ac.uk. RNAseq data are available at Array Express under accession numbers E-MTAB-4079 and E-MTAB-4026. Processed RNAseq data are also available at http://gastrulation.stemcells.cam.ac.uk/scialdone2016.
References
Lawson, K. A., Meneses, J. J. & Pedersen, R. A. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development 113, 891–911 (1991)
Van Handel, B. et al. Scl represses cardiomyogenesis in prospective hemogenic endothelium and endocardium. Cell 150, 590–605 (2012)
Org, T. et al. Scl binds to primed enhancers in mesoderm to regulate hematopoietic and cardiac fate divergence. EMBO J. 34, 759–777 (2015)
Ema, M. et al. Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, PECAM-1, Tie2, endoglin, and CD34 in the mouse embryo. Blood 108, 4018–4024 (2006)
Mikkola, H. K. A., Fujiwara, Y., Schlaeger, T. M., Traver, D. & Orkin, S. H. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood 101, 508–516 (2003)
Wilkinson, D. G., Bhatt, S. & Herrmann, B. G. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature 343, 657–659 (1990)
Burtscher, I. & Lickert, H. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development 136, 1029–1038 (2009)
Chintala, S. et al. The Slc35d3 gene, encoding an orphan nucleotide sugar transporter, regulates platelet-dense granules. Blood 109, 1533–1540 (2007)
Henke, C. et al. Selective expression of sense and antisense transcripts of the sushi-ichi-related retrotransposon – derived family during mouse placentogenesis. Retrovirology 12, 9 (2015)
Tam, P. P. L. & Zhou, S. X. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev. Biol. 178, 124–132 (1996)
Solnica-Krezel, L. & Sepich, D. S. Gastrulation: making and shaping germ layers. Annu. Rev. Cell Dev. Biol. 28, 687–717 (2012)
Kitajima, S., Takagi, A., Inoue, T. & Saga, Y. MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development 127, 3215–3226 (2000)
Rozbicki, E. et al. Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation. Nature Cell Biol. 17, 397–408 (2015)
Haghverdi, L., Buettner, F. & Theis, F. J. Diffusion maps for high-dimensional single-cell analysis of differentiation data. Bioinformatics 31, 2989–2998 (2015)
Moignard, V. et al. Decoding the regulatory network of early blood development from single-cell gene expression measurements. Nature Biotechnol. 33, 269–276 (2015)
Saga, Y. Segmental border is defined by the key transcription factor Mesp2, by means of the suppression of Notch activity. Dev. Dyn. 236, 1450–1455 (2007)
Lawson, K. A. et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424–436 (1999)
Fujiwara, Y., Browne, C. P., Cunniff, K., Goff, S. C. & Orkin, S. H. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl Acad. Sci. USA 93, 12355–12358 (1996)
Lancrin, C. et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457, 892–895 (2009)
Padrón-Barthe, L. et al. Clonal analysis identifies hemogenic endothelium and not hemangioblasts as the source of the blood-endothelial common lineage in the mouse embryo. Blood 124, 2523–2532 (2014)
Tam, P. P., Parameswaran, M., Kinder, S. J. & Weinberger, R. P. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development 124, 1631–1642 (1997)
Porcher, C. et al. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell 86, 47–57 (1996)
Shivdasani, R. A., Mayer, E. L. & Orkin, S. H. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 373, 432–434 (1995)
Batta, K., Florkowska, M., Kouskoff, V. & Lacaud, G. Direct reprogramming of murine fibroblasts to hematopoietic progenitor cells. Cell Reports 9, 1871–1884 (2014)
Chen, J. Y. et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature 530, 223–227 (2016)
Bheda, P. & Schneider, R. Epigenetics reloaded: the single-cell revolution. Trends Cell Biol. 24, 712–723 (2014)
Achim, K. et al. High-throughput spatial mapping of single-cell RNA-seq data to tissue of origin. Nature Biotechnol. 33, 503–509 (2015)
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nature Biotechnol. 33, 495–502 (2015)
Robertson, E. J. Dose-dependent Nodal/Smad signals pattern the early mouse embryo. Semin. Cell Dev. Biol. 32, 73–79 (2014)
Downs, K. M. & Davies, T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development 118, 1255–1266 (1993)
Wilkinson, A. C. et al. Single site-specific integration targeting coupled with embryonic stem cell differentiation provides a high-throughput alternative to in vivo enhancer analyses. Biol. Open 2, 1229–1238 (2013)
Elefanty, A. G. et al. Characterization of hematopoietic progenitor cells that express the transcription factor SCL, using a lacZ “knock-in” strategy. Proc. Natl Acad. Sci. USA 95, 11897–11902 (1998)
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nature Protocols 9, 171–181 (2014)
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015)
Stegle, O., Teichmann, S. A. & Marioni, J. C. Computational and analytical challenges in single-cell transcriptomics. Nature Rev. Genet. 16, 133–145 (2015)
van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008)
van der Maaten, L. Barnes-Hut-SNE. Preprint at http://arxiv.org/pdf/1301.3342 (2013)
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010)
Brennecke, P. et al. Accounting for technical noise in single-cell RNA-seq experiments. Nature Methods 10, 1093–1095 (2013)
Buettner, F. et al. Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Nature Biotechnol. 33, 155–160 (2015)
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010)
Langfelder, P., Zhang, B. & Horvath, S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 24, 719–720 (2008)
Halkidi, M., Batistakis, Y. & Vazirgiannis, M. On clustering validation techniques. J. Intell. Inf. Syst. 17, 107–145 (2001)
Rousseeuw, P. J. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 20, 53–65 (1987)
Angerer, P. et al. destiny – diffusion maps for large-scale single-cell data in R. bioRxivhttp://dx.doi.org/10.1101/023309 (2015)
Burnham, K. P. & Anderson, D. R. in Model Selection and Multimodel Inference A Practical Information-Theoretic Approach 2nd edn, Ch. 2 (Springer, 2002)
Breiman, L. Random Forests. Mach. Learn. 45, 5–32 (2001)
Liaw, A. & Wiener, M. Classification and Regression by randomForest. R News 2, 18–22 (2002)
Bryja, V., Bonilla, S. & Arenas, E. Derivation of mouse embryonic stem cells. Nature Protocols 1, 2082–2087 (2006)
Tanaka, Y. et al. Circulation-independent differentiation pathway from extraembryonic mesoderm toward hematopoietic stem cells via hemogenic angioblasts. Cell Reports 8, 31–39 (2014)
Sroczynska, P., Lancrin, C., Pearson, S., Kouskoff, V. & Lacaud, G. In vitro differentiation of mouse embryonic stem cells as a model of early hematopoietic development. Methods Mol. Biol. 538, 317–334 (2009)
Wilson, N. K. et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell 7, 532–544 (2010)
Zwart, W. et al. A carrier-assisted ChIP-seq method for estrogen receptor-chromatin interactions from breast cancer core needle biopsy samples. BMC Genomics 14, 232 (2013)
Sánchez-Castillo, M. et al. CODEX: a next-generation sequencing experiment database for the haematopoietic and embryonic stem cell communities. Nucleic Acids Res. 43, D1117–D1123 (2015)
Zhang, Y. et al. Model-based analysis of ChIP-seq (MACS). Genome Biol. 9, R137 (2008)
Wu, H. & Ji, H. PolyaPeak: detecting transcription factor binding sites from ChIP-seq using peak shape information. PLoS ONE 9, e89694 (2014)
Wilkinson, D. G. In Situ Hybridization (Oxford Univ. Press, 1999)
Beddington, R. S. & Robertson, E. J. Axis development and early asymmetry in mammals. Cell 96, 195–209 (1999)
Du, J. et al. O-fucosylation of thrombospondin type 1 repeats restricts epithelial to mesenchymal transition (EMT) and maintains epiblast pluripotency during mouse gastrulation. Dev. Biol. 346, 25–38 (2010)
Donnison, M. et al. Loss of the extraembryonic ectoderm in Elf5 mutants leads to defects in embryonic patterning. Development 132, 2299–2308 (2005)
Mitsunaga, K. et al. Loss of PGC-specific expression of the orphan nuclear receptor ERR-β results in reduction of germ cell number in mouse embryos. Mech. Dev. 121, 237–246 (2004)
Baldwin, H. S. et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development 120, 2539–2553 (1994)
Naiche, L. A., Arora, R., Kania, A., Lewandoski, M. & Papaioannou, V. E. Identity and fate of Tbx4-expressing cells reveal developmental cell fate decisions in the allantois, limb, and external genitalia. Dev. Dyn. 240, 2290–2300 (2011)
Tamplin, O. J. et al. Microarray analysis of Foxa2 mutant mouse embryos reveals novel gene expression and inductive roles for the gastrula organizer and its derivatives. BMC Genomics 9, 511 (2008)
Vincent, S. D. et al. Prdm1 functions in the mesoderm of the second heart field, where it interacts genetically with Tbx1, during outflow tract morphogenesis in the mouse embryo. Hum. Mol. Genet. 23, 5087–5101 (2014)
Morkel, M. et al. β-Catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development 130, 6283–6294 (2003)
Niwa, H., Miyazaki, J. & Smith, A. G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nature Genet. 24, 372–376 (2000)
Pearce, J. J. H. & Evans, M. J. Mml, a mouse Mix-like gene expressed in the primitive streak. Mech. Dev. 87, 189–192 (1999)
Pennisi, D. et al. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nature Genet. 24, 434–437 (2000)
Gordon, E. J., Gale, N. W. & Harvey, N. L. Expression of the hyaluronan receptor LYVE-1 is not restricted to the lymphatic vasculature; LYVE-1 is also expressed on embryonic blood vessels. Dev. Dyn. 237, 1901–1909 (2008)
Kallianpur, A. R., Jordan, J. E. & Brandt, S. J. The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis. Blood 83, 1200–1208 (1994)
Robb, L. et al. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc. Natl Acad. Sci. USA 92, 7075–7079 (1995)
Tanaka, Y. et al. The transcriptional programme controlled by Runx1 during early embryonic blood development. Dev. Biol. 366, 404–419 (2012)
North, T. et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126, 2563–2575 (1999)
Palis, J., McGrath, K. E. & Kingsley, P. D. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood 86, 156–163 (1995)
Drissen, R. et al. The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability. Mol. Cell. Biol. 25, 5205–5214 (2005)
Southwood, C. M., Downs, K. M. & Bieker, J. J. Erythroid Krüppel-like factor exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Dev. Dyn. 206, 248–259 (1996)
Silver, L. & Palis, J. Initiation of murine embryonic erythropoiesis: a spatial analysis. Blood 89, 1154–1164 (1997)
Lanctôt, C., Lamolet, B. & Drouin, J. The bicoid-related homeoprotein Ptx1 defines the most anterior domain of the embryo and differentiates posterior from anterior lateral mesoderm. Development 124, 2807–2817 (1997)
Lania, G., Ferrentino, R. & Baldini, A. TBX1 represses Vegfr2 gene expression and enhances the cardiac fate of VEGFR2+ cells. PLoS ONE 10, e0138525 (2015)
Brown, C. B. et al. Cre-mediated excision of Fgf8 in the Tbx1 expression domain reveals a critical role for Fgf8 in cardiovascular development in the mouse. Dev. Biol. 267, 190–202 (2004)
Brennan, J. et al. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411, 965–969 (2001)
Meno, C. et al. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol. Cell 4, 287–298 (1999)
Bessho, Y. et al. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev. 15, 2642–2647 (2001)
Oginuma, M., Niwa, Y., Chapman, D. L. & Saga, Y. Mesp2 and Tbx6 cooperatively create periodic patterns coupled with the clock machinery during mouse somitogenesis. Development 135, 2555–2562 (2008)
Forlani, S., Lawson, K. A. & Deschamps, J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development 130, 3807–3819 (2003)
Zeigler, B. M. et al. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development 133, 4183–4192 (2006)
Downs, K. M., Hellman, E. R., McHugh, J., Barrickman, K. & Inman, K. E. Investigation into a role for the primitive streak in development of the murine allantois. Development 131, 37–55 (2004)
Caprioli, A., Jaffredo, T., Gautier, R., Dubourg, C. & Dieterlen-Lièvre, F. Blood-borne seeding by hematopoietic and endothelial precursors from the allantois. Proc. Natl Acad. Sci. USA 95, 1641–1646 (1998)
van Nes, J. et al. The Cdx4 mutation affects axial development and reveals an essential role of Cdx genes in the ontogenesis of the placental labyrinth in mice. Development 133, 419–428 (2006)
Yang, J. T., Rayburn, H. & Hynes, R. O. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development 121, 549–560 (1995)
Solloway, M. J. & Robertson, E. J. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development 126, 1753–1768 (1999)
Drake, C. J. & Fleming, P. A. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 95, 1671–1679 (2000)
Lee, D. et al. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell 2, 497–507 (2008)
Carapuço, M., Nóvoa, A., Bobola, N. & Mallo, M. Hox genes specify vertebral types in the presomitic mesoderm. Genes Dev. 19, 2116–2121 (2005)
Zhang, H. et al. Expression of podocalyxin separates the hematopoietic and vascular potentials of mouse embryonic stem cell-derived mesoderm. Stem Cells 32, 191–203 (2014)
Herrmann, B. G. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development 113, 913–917 (1991)
Weidgang, C. E. et al. TBX3 directs cell-fate decision toward mesendoderm. Stem Cell Rep. 1, 248–265 (2013)
Perea-Gómez, A., Shawlot, W., Sasaki, H., Behringer, R. R. & Ang, S. HNF3β and Lim1 interact in the visceral endoderm to regulate primitive streak formation and anterior-posterior polarity in the mouse embryo. Development 126, 4499–4511 (1999)
Saga, Y. et al. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development 126, 3437–3447 (1999)
Trimborn, T., Gribnau, J., Grosveld, F. & Fraser, P. Mechanisms of developmental control of transcription in the murine α- and β-globin loci. Genes Dev. 13, 112–124 (1999)
Kingsley, P. D., Malik, J., Fantauzzo, K. A. & Palis, J. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood 104, 19–25 (2004)
Hodge, D. et al. A global role for EKLF in definitive and primitive erythropoiesis. Blood 107, 3359–3370 (2006)
Isern, J. et al. Single-lineage transcriptome analysis reveals key regulatory pathways in primitive erythroid progenitors in the mouse embryo. Blood 117, 4924–4934 (2011)
Joshi, A., Hannah, R., Diamanti, E. & Göttgens, B. Gene set control analysis predicts hematopoietic control mechanisms from genome-wide transcription factor binding data. Exp. Hematol. 41, 354–366.e14 (2013)
Goode, D. K. et al. Dynamic gene regulatory networks drive hematopoietic specification and differentiation. Dev. Cell 36, 572–587 (2016)
Acknowledgements
We thank M. de Bruijn, A. Martinez-Arias, J. Nichols and C. Mulas for discussion, the Cambridge Institute for Medical Research Flow Cytometry facility for their expertise in single-cell index sorting, and S. Lorenz from the Sanger Single Cell Genomics Core for supervising purification of Tal1−/− sequencing libraries. ChIP-seq reads were processed by R. Hannah. Research in the authors’ laboratories is supported by the Medical Research Council, Cancer Research UK, the Biotechnology and Biological Sciences Research Council, Bloodwise, the Leukemia and Lymphoma Society, and the Sanger-EBI Single Cell Centre, and by core support grants from the Wellcome Trust to the Cambridge Institute for Medical Research and Wellcome Trust - MRC Cambridge Stem Cell Institute and by core funding from Cancer Research UK and the European Molecular Biology Laboratory. Y.T. was supported by a fellowship from the Japan Society for the Promotion of Science. W.J. is a Wellcome Trust Clinical Research Fellow. A.S. is supported by the Sanger-EBI Single Cell Centre. This work was funded as part of Wellcome Trust Strategic Award 105031/D/14/Z ‘Tracing early mammalian lineage decisions by single-cell genomics’ awarded to W. Reik, S. Teichmann, J. Nichols, B. Simons, T. Voet, S. Srinivas, L. Vallier, B. Göttgens and J. Marioni.
Author information
Authors and Affiliations
Contributions
A.S. and W.J. processed and analysed single-cell RNA sequencing (RNA-seq) data. A.S. and V.M. generated figures. Y.T. and W.J. performed embryo dissection. N.K.W., V.M. and I.C.M. performed single-cell RNA-seq experiments. Y.T. performed flow cytometry, ESC differentiation and in situ hybridization. V.M. performed ChIP-seq assays. A.S., W.J., Y.T., V.M., B.G. and J.C.M. interpreted results and wrote the paper. B.G. and J.C.M. supervised and conceived the study.
Corresponding authors
Additional information
Reviewer Information Nature thanks A.-K. Hadjantonakis, P. Robson and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 FACS of single cells.
a, Flk1+ cells were sorted from three embryos at primitive streak and head fold stages and four embryos at neural plate stage. Labels such as ‘S4’ refer to the embryo number in the metadata available online at http://gastrulation.stemcells.cam.ac.uk/scialdone2016. b, CD41+Flk1− cells (red gate) and CD41+Flk1+ (green gate) cells were sorted from eight embryos each at neural plate and head fold stages (see Fig. 1 for cell numbers). Each stage was sorted on two occasions. Labels above FACS plots refer to the sort and embryo number in the metadata available online, as above. In all plots, pink text indicates the percentage of cells in that gate.
Extended Data Figure 2 Quality control of single-cell RNA-seq data.
a, Table showing numbers and estimates of numbers of cells of different phenotypes present in embryos between E6.5 and E7.75 (head fold stage) and numbers sorted for this study. *Total cell numbers for E6.5 are from Beddington and Robertson (1999)58. †Total numbers and numbers of Flk1+ cells are from Moignard et al., (2015)15. Percentages of cells expressing Flk1 and/or CD41 at neural plate and head fold stages are the average values from the embryos used in this study and were used to calculate the estimated numbers present in embryos from the total cell numbers. ND, not done. b, t-SNE representation of the five metrics used to assess the quality of all 2,208 sorted cells from the wild-type and Tal1 experiments. Only cells that passed all criteria (blue circles) were used for downstream analysis. c, Squared coefficient of variation (CV2) as a function of the mean normalized counts (μ) for genes across all cells. The green line shows the fit CV2 = a1/μ + α0. All highly variable genes (with an adjusted P value < 0.1) are marked by red circles. d, Number of genes detected (that is, with more than ten normalized read counts) in cells across the different clusters in the WT (left) and the Tal1−/− (right) mice. Boxes indicate the median and interquartile range.
Extended Data Figure 3 Identifying cell clusters.
The dynamic hybrid cut algorithm was used with all possible values of the ‘deepSplit’ parameter on 100 bootstrapped subsamples. a, b, To assess the quality of the clustering, the Pearson gamma (a) and the average silhouette width (b) were calculated. Higher values of these parameters correspond to better clustering. The Pearson gamma represents the correlation between the dissimilarity of samples and a binary variable that equals 0 for pairs of samples in the same cluster and 1 for samples in different clusters. The average silhouette width measures the average separation between neighbouring clusters43,44. At ‘deepSplit’ = 2 the Pearson gamma is highest whereas the average silhouette width begins to decrease. This suggests that at such a value of the ‘deepSplit’ parameter a good compromise between robustness and sensitivity is achieved. The Pearson gamma and the average silhouette width were computed with the R function ‘cluster.stats’ in the ‘fpc’ package (version 2.1-9). c–f, Examples of marker genes for four clusters: Mesp1 for cluster 4 (top-ranked marker) (c), Hbb-bh1 for cluster 8 (fourth-ranked) (d), Alx1 for cluster 10 (top-ranked) (e) and Sox18 for cluster 6 (second-ranked) (f). The y axis shows the log10-normalized expression of the genes. For a–f, boxes indicate the median and interquartile range. g, Dendrogram showing the clustering of the cells in the first experiment. The colours at the bottom indicate the cluster each cell was assigned to by the dynamic hybrid cut algorithm. Cluster assignment was used to sort cells in Fig. 1b. h, Identities were assigned to the ten clusters in Fig. 1c on the basis of the expression of key genes20,50,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80 associated with various mesodermal lineages or spatial locations within the embryo.
Extended Data Figure 4 Expression of key marker genes in E7.0–7.75 embryos.
Schematic representations of expression patterns were generated from published in situ hybridization data (see citations) for key markers of clusters 1 (magenta, visceral endoderm59), 2 (pink, extra-embryonic ectoderm61), 6 (red, yolk sac endothelium62), 9 (black, allantois63,64) and 10 (green, second heart field65,81). Anterior is shown on the left and posterior on the right. Also shown is the t-SNE for all 1,205 cells or 682 cells from E7.0 onwards (primitive streak, neural plate and head fold stages) indicating expression of each gene (white, low; purple, high).
Extended Data Figure 5 Expression of key genes used for sorting single cells.
a, t-SNE as in Fig. 1 showing the sorting strategy for each of the 1,205 cells. b, Expression of Flk1 (Kdr), CD41 (Itga2b), Scl (Tal1), Gata2 and T (Brachyury) superimposed onto the t-SNE. c, t-SNE showing only the 682 cells from primitive streak, neural plate and head fold stages, coloured according to cluster as in Fig. 1c, e. d, t-SNE for the 481 E6.5 cells in cluster 3, as in Fig. 2a. Each point is coloured by expression of T and Foxa2.
Extended Data Figure 6 Pseudospace analysis of cluster 4 correlates with anterior–posterior position along the primitive streak.
a, Diffusion plot of the 216 cells in cluster 4. Different colours correspond to different plates and different lanes of flow cells. b, Table showing the number of cells in each stage analysed on the different lanes of flow cells (S, primitive streak; NP, neural plate; HF, head fold). c, A direction in the diffusion space can be identified by the angle α that it forms with the first diffusion component (left panel). For each value of α the right panel shows the percentage of variance explained by the batch effect associated to plates SLX-8408 and SLX-8409 (orange line) and plates SLX-8410 and SLX-8411 (blue line). Labels α1 and α2 are the angles corresponding to directions that correlate the least with the batch effect (that is, variance explained by the batch effect is minimum). d, The use of alternative dimensionality reduction techniques results in the identification of highly correlated pseudospace coordinates. A t-SNE projection of the dissimilarity matrix was performed (perplexity set to 50), and the direction corresponding to the pseudospace coordinate was estimated by minimizing the correlation with the batch effect (left panel; Spearman correlation between the two pseudospace coordinates 0.79, P < 2.2 × 10−16). Independent component analysis was performed on the dissimilarity matrix with the ‘fastICA’ R function, and three independent components (corresponding to the two batch effects and the biological effect) were estimated. The presumptive pseudospace coordinate is the component having the smallest correlation with the batch effects (right panel; Spearman correlation coefficient is 0.97, P < 2.2 × 10−16). e, Plots showing the average expression of genes in clusters 1–3 of Fig. 3c along the pseudospace axis. Gene expression levels are normalized between 0 and 1. Dark red lines indicate the normalized mean expression levels of genes in each cluster as obtained from the fitting procedure and red shaded area indicates standard deviation. f, Expression of T as function of the pseudospace coordinate. g, Gene expression levels for example genes showing high-low-high expression pattern across the blue cluster. In f and g, putative anterior cells are to the left and posterior to the right. Each dot represents a cell and red lines indicate fits based on local polynomial functions (see Methods). h, We performed principal component analysis on the cells in cluster 4 by using markers of pre-somitic mesoderm as anterior mesoderm markers and genes expressed in haemato-vascular and allantoic mesoderm as posterior markers82,83,84,85,86,87,88,89,90,91,92,93,94,95, as well as Podxl which was shown to separate distinct Flk1+ mesodermal lineages96. The first component explained 36% of the total variance and was highly correlated with the pseudospace coordinate (left; Spearman rank correlation 0.84, P < 2.2 × 10−16). All the anterior markers were negatively correlated with the pseudospace coordinate, whereas all posterior markers had a positive correlation (right).
Extended Data Figure 7 Expression of key genes along the anterior–posterior axis of the primitive streak in E7.0–7.75 embryos.
Schematic representations of gene expression were generated from published in situ hybridization data (see citations) for key markers of clusters 4 (blue, mesoderm) and 7 (yellow, posterior mesoderm/blood progenitors). Expression of T (Brachyury)97 and Flk1 (Kdr, from in-house data) are shown to illustrate the extent of the primitive streak at E7.5. Lefty2 and Tbx6 (ref. 59) are expressed in the putative anterior portion of cluster 4 and in more anterior regions of the primitive streak in in situ analysis. Tbx3 (ref. 98) and Bmp4 (ref. 99) are expressed in the more posterior portion of cluster 4 and in the embryo are expressed in the more posterior region of the primitive streak around the amnion and into the extra-embryonic mesoderm. Tek and Fli1 (from in-house data) are expressed in cluster 7 and in the embryo are found exclusively in the extra-embryonic portion. Also shown is the t-SNE for the cells from E7.0 onwards (primitive streak, neural plate and head fold stages) indicating expression of each gene (white, low; purple, high).
Extended Data Figure 8 Pseudotime analysis of primitive erythroid development.
a, Diffusion plot of the 271 cells in clusters 7 and 8. Different colours correspond to different plates and lanes of flow cells. b, Table showing the number of cells in each stage collected on the different plates (S, primitive streak; NP, neural plate; HF, head fold). c, Analogously to Extended Data Fig. 6, the angle α identifies a direction in the diffusion space (left panel). The percentage of variance explained by the batch effect associated to plates SLX-8344 and SLX-8345 is plotted as a function of α in the right panel. d, The pseudotime coordinate is robust to the use of different dimensionality reduction techniques, as shown in the left panel with t-SNE (Spearman correlation 0.92, P < 2.2 × 10−16) and in the right panel with independent component analysis (Spearman correlation 0.97, P < 2.2 × 10−16; same procedure described in Extended Data Fig. 6d). e, Plots showing the average expression of genes in clusters 1–3 of Fig. 4c along the pseudotime axis. Gene expression levels are normalized between 0 and 1. Dark red lines are the average expression levels of genes in each cluster as obtained from the fitting procedure, after normalization. Red shaded areas indicate standard deviation. f, Principal component analysis was performed on the expression pattern of genes known from previous studies to be upregulated or downregulated along the blood developmental trajectory15,66,100,101,102,103,104. The first principal component (explaining 44% of total variance) showed a very strong correlation with the pseudotime coordinate (left; Spearman correlation coefficient 0.91, P < 2.2 × 10−16). All upregulated (downregulated) genes positively (negatively) correlate with the pseudotime coordinate (right).
Extended Data Figure 9 ChIP-seq for Gata1 in ESC-derived haematopoietic cells.
a, Flow cytometry for Gata1–mCherry and Runx1-IRES–GFP knock-in reporter genes in embryoid body cells after 5 days of haematopoietic differentiation. Cells were sorted for the expression of both Runx1-IRES–GFP and Gata1–mCherry knock-in reporter genes to provide in vitro equivalents of the developing primitive erythrocytes assayed by RNA-seq. The gate used for sorting is shown in red. b, Numbers of reads and peaks identified for Gata1 and an input sample after mapping and peak calling; 4,135 Gata1 peaks were identified. c, Distribution of Gata1 peaks between promoter, intragenic and intergenic sequences. d, University of California, Santa Cruz Genome Browser tracks for Gata1 and input sample at the Zfpm1 (Fog1) locus known to be a target of Gata1, indicating the quality of the ChIP-seq data. e, Expression of Gata1 target Zfpm1 during the pseudotimecourse for erythroid development, as in Fig. 4.
Extended Data Figure 10 Collection of embryos from Tal1 LacZ/+ crosses.
a, Genotyping PCR for embryos from Tal1 LacZ/+ crosses. Lower band is the WT allele and upper band is the mutant allele carrying a neomycin knock in. Presence of both bands indicates heterozygosity. Embryos from which sequencing data were obtained are indicated with a red star and the number given corresponds to embryo identity in the metadata available online with the sequencing data. b, t-SNE as in Fig. 5d showing Tal1 data (triangles; 377 cells) and original WT data (grey circles; 1,205 cells). Tal1 data are coloured according to the embryo stage from which they were collected: green, neural plate; red, head fold; orange, four-somite pair. c, As in Fig. 5d, showing the complete list of genes. d, Gene set control analysis105 was used to identify statistically significant overlaps between genes significantly downregulated in Tal1−/− compared with WT cells in the endothelial cluster (see Fig. 5) and Tal1 targets identified by ChIP-seq. Gene set control analysis identified an enrichment of our gene set with Tal1 ChIP-seq in ESC-derived haemangioblasts and haemogenic endothelium106, but not in ESC-derived haematopoietic progenitors106 or a haematopoietic progenitor cell line52.
Supplementary information
Supplementary Table 1
Genes correlated with T in E6.5 epiblast cells. Correlation was calculated using Spearman rank correlation. All genes significantly correlated at FDR <0.1 are listed. (XLSX 138 kb)
Supplementary Table 2
Gene ontology terms for genes dynamically expressed across pseudospace and pseudotime in Figures 2 and 4. Significant GO terms associated with the gene clusters in Figure 3c and Figure 4c (both p-value <10-4). In both cases, no significant GO terms were associated with the smallest cluster of transient expressed genes, likely due to the low numbers of genes in these clusters. (XLSX 34 kb)
Supplementary Table 3
Full list of marker genes associated with clusters in Figures 1 and Extended Data Fig. 2. (XLSX 77 kb)
Supplementary Table 4
List of genes differentially expressed along the pseudo-space trajectory shown in Figure 3. The three clusters of genes differentially expressed along the pseudo-space trajectory are listed (see Figures 3 and Extended Data Fig. 5) with their ΔAIC scores (see Methods). (XLSX 65 kb)
Supplementary Table 5
List of genes differentially expressed along the pseudo-time trajectory shown in Figure 4. The three clusters of genes differentially expressed along the pseudo-time trajectory are listed (see Figures 4 and Extended Data Fig. 8), along with their ΔAIC scores (see Methods). (XLSX 83 kb)
Supplementary Table 6
Lists of genes bound by Gata in ChIP-seq experiment, and differentially expressed along the pseudo-time trajectory shown in Figure 4 and bound by Gata1. (XLSX 98 kb)
Supplementary Table 7
List of 319 genes up-regulated in endothelium (red, cluster 6) and blood cells (brown, cluster 8) when compared to nascent mesoderm population (blue, cluster 4) in Figure 5d. (XLSX 73 kb)
Supplementary Table 8
List of genes differentially expressed between Tal1-/- endothelium and WT endothelium in Figure 5e. Genes up-regulated in Tal1-/- mice correspond to fold-changes greater than 1. (XLSX 56 kb)
Supplementary Table 9
Genes up- and down-regulated by Tal1. Gene sets used in Figure 5e, taken from Org et al., 20153 Supplementary Tables 1c and 1d. (XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Scialdone, A., Tanaka, Y., Jawaid, W. et al. Resolving early mesoderm diversification through single-cell expression profiling. Nature 535, 289–293 (2016). https://doi.org/10.1038/nature18633
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18633