Abstract
Cancer is a disease of ageing. Clinically, aged cancer patients tend to have a poorer prognosis than young. This may be due to accumulated cellular damage, decreases in adaptive immunity, and chronic inflammation. However, the effects of the aged microenvironment on tumour progression have been largely unexplored. Since dermal fibroblasts can have profound impacts on melanoma progression1,2,3,4, we examined whether age-related changes in dermal fibroblasts could drive melanoma metastasis and response to targeted therapy. Here we find that aged fibroblasts secrete a Wnt antagonist, sFRP2, which activates a multi-step signalling cascade in melanoma cells that results in a decrease in β-catenin and microphthalmia-associated transcription factor (MITF), and ultimately the loss of a key redox effector, APE1. Loss of APE1 attenuates the response of melanoma cells to DNA damage induced by reactive oxygen species, rendering the cells more resistant to targeted therapy (vemurafenib). Age-related increases in sFRP2 also augment both angiogenesis and metastasis of melanoma cells. These data provide an integrated view of how fibroblasts in the aged microenvironment contribute to tumour progression, offering new possibilities for the design of therapy for the elderly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
07 August 2023
Editor’s Note: "Readers are alerted that the reliability of some of the data presented in this manuscript is currently in question. Appropriate editorial action will be taken once this matter is resolved."
References
Ruiter, D., Bogenrieder, T., Elder, D. & Herlyn, M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 3, 35–43 (2002)
Li, G., Satyamoorthy, K. & Herlyn, M. Dynamics of cell interactions and communications during melanoma development. Crit. Rev. Oral Biol. Med. 13, 62–70 (2002)
Hsu, M. Y., Meier, F. & Herlyn, M. Melanoma development and progression: a conspiracy between tumor and host. Differentiation 70, 522–536 (2002)
Bogenrieder, T. & Herlyn, M. Cell-surface proteolysis, growth factor activation and intercellular communication in the progression of melanoma. Crit. Rev. Oncol. Hematol. 44, 1–15 (2002)
Dankort, D. et al. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nature Genet. 41, 544–552 (2009)
Hoek, K. S. MITF: the power and the glory. Pigment Cell Melanoma Res. 24, 262–263 (2011)
Hoek, K. S. et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 68, 650–656 (2008)
Webster, M. R., Kugel, C. H., III & Weeraratna, A. T. The Wnts of change: how Wnts regulate phenotype switching in melanoma. Biochim. Biophys. Acta 1856, 244–251 (2015)
O’Connell, M. P. et al. Hypoxia induces phenotypic plasticity and therapy resistance in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer Discov. 3, 1378–1393 (2013)
Flach, E. H., Rebecca, V. W., Herlyn, M., Smalley, K. S. & Anderson, A. R. Fibroblasts contribute to melanoma tumor growth and drug resistance. Mol. Pharm. 8, 2039–2049 (2011)
Campisi, J. The role of cellular senescence in skin aging. J. Investig. Dermatol. Symp. Proc. 3, 1–5 (1998)
Coppé, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010)
Park, H. W. Biological aging and social characteristics: gerontology, the Baltimore city hospitals, and the national institutes of health. J. Hist. Med. Allied Sci. 68, 49–86 (2013)
Arozarena, I. et al. In melanoma, beta-catenin is a suppressor of invasion. Oncogene 30, 4531–4543 (2011)
Chien, A. J. et al. Activated Wnt/β-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc. Natl Acad. Sci. USA 106, 1193–1198 (2009)
Bouchlaka, M. N. et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J. Exp. Med. 210, 2223–2237 (2013)
Lento, W. et al. Loss of β-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes Dev. 28, 995–1004 (2014)
Widlund, H. R. et al. β-Catenin-induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J. Cell Biol. 158, 1079–1087 (2002)
Liu, F., Fu, Y. & Meyskens, F. L. Jr. MiTF regulates cellular response to reactive oxygen species through transcriptional regulation of APE-1/Ref-1. J. Invest. Dermatol. 129, 422–431 (2009)
Corazao-Rozas, P. et al. Mitochondrial oxidative stress is the achille’s heel of melanoma cells resistant to Braf-mutant inhibitor. Oncotarget 4, 1986–1998 (2013)
Yu, L. et al. Involvement of superoxide and nitric oxide in BRAF inhibitor PLX4032-induced growth inhibition of melanoma cells. Integr. Biol. 350, 1391–1396 (2014)
Biechele, T. L. et al. Wnt/β-catenin signaling and AXIN1 regulate apoptosis triggered by inhibition of the mutant kinase BRAFV600E in human melanoma. Sci. Signal. 5, ra3 (2012)
Konieczkowski, D. J. et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov. 4, 816–827 (2014)
Yun, J. et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350, 1391–1396 (2015)
Issa, J. P. CpG-island methylation in aging and cancer. Curr. Top. Microbiol. Immunol. 249, 101–118 (2000)
Issa, J. P. Aging and epigenetic drift: a vicious cycle. J. Clin. Invest. 124, 24–29 (2014)
Shah, P. P. et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 27, 1787–1799 (2013)
Viros, A. et al. Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TP53. Nature 511, 478–482 (2014)
Bald, T. et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 507, 109–113 (2014)
Berking, C. & Herlyn, M. Human skin reconstruct models: a new application for studies of melanocyte and melanoma biology. Histol. Histopathol . 16, 669–674 (2001)
Acknowledgements
We thank D. Altieri, R. Marais, Z. Ronai, M. McMahon, and B. Vogelstein for comments on the manuscript. We thank R. Somasundaram for advice on immune analyses, M. Herlyn for the WM cell lines, and G. Bollag for PLX4720. We also thank R. Delgiacco, D. Gourevitch, F. Keeney, and D. Schultz. We thank A. Dias-Wanigasekera, E. Gaddy, and M. Ha for technical assistance, and R. Locke for editing the manuscript. This work was supported in part by funds from the Intramural Program of the National Institute on Aging, Baltimore, Maryland (N.M., K.G.B., R.M., W.H.W., L.F.), The Harry J. Lloyd Foundation (K.M., A.T.W.), P01 CA 114046-06 (A.T.W., Q.L.), T32 CA 9171-36 (M.R.W., C.H.K.), an ACS-IRG award (A.T.W.), the Melanoma Research Foundation (A.T.W.), and RO1 CA174746-01 (A.T.W., A.K.). Core facilities at the Wistar are supported by Cancer Center Support Grant P30 CA010815.
Author information
Authors and Affiliations
Contributions
A.T.W. conceived and designed the project. A.T.W. and A.K. designed and supervised the experiments. A.K., M.R.W., K.M., R.B., A.N., C.H.K., V.M.D., J.A., M.P.O., P.C., A.A.V., W.H.W., E.L., and K.M.A. performed the experiments. A.T.W., A.K., A.V.K., H.Y.T., X.Y., E.L., Z.E., K.G.B., R.Z., X.X., Q.L., and D.W.S. analysed the experimental data. A.T.W., A.K., and Q.L. designed and supervised data analysis and statistical analysis. M.B., A.R., D.S., J.S., B.S., R.S.L., M.C., A.M.M., G.V.L., D.B.J., R.M., N.B.M., L.F., K.M., K.T.F., D.T.F., J.A.W., Z.A.C., M.T.T., C.H., E.B., and G.K. performed data collection and provided anonymized patient data and samples and reagents. A.T.W. and A.K. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.T.F. is a consultant to GlaxoSmithKline, Roche, and Novartis; G.V.L. is a consultant to Amgen, Bristol-Myers Squibb, GlaxoSmithKline, MSD, Novartis, and Roche; J.A.W. is a consultant to Roche and GlaxoSmithKline. We do not believe these relationships have any direct impact on this work.
Extended data figures and tables
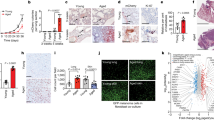
Extended Data Figure 1 Characterization of young and aged fibroblasts.
a, One million Yumm1.7 cells were injected into the tail vein of young (8 weeks, n = 10 mice) and aged (52 weeks, n = 10 mice) mice; 3 weeks later, lungs were assessed for metastatic colonies. Samples were analysed by haematoxylin and eosin staining. Number of mice with metastatic colonies in the lungs is quantified in the graph. b, Proliferation rate of aged and young fibroblasts was measured by simple cell counts over a period of 12 days. ANOVA is insignificant (P = 0.234). c, Young and aged fibroblasts were assessed for basal β-galactosidase activity after five passages in culture. Representative images from two cell lines are shown for young and aged fibroblasts, original magnification ×100. d, Staining of fibroblasts in skin reconstructs with α-SMA-1 to assess persistence of fibroblasts in cell culture. Representative images, original magnification ×150. e, WM793 melanoma cells were grown in organotypic three-dimensional skin reconstructs built with three different fibroblast cell lines derived from healthy young (25–35 years) and healthy aged (55–65 years) individuals. Representative images, original magnification ×150. Invasion was quantified using NIS Element software. ANOVA was performed (P = 0.007). Holm–Šídák multiple comparisons comparing each young cell line with each aged cell line indicated P < 0.05. f, WM793 melanoma cells exposed to conditioned media from young and aged fibroblasts were assessed for proliferation using simple cell counts. Repeated measures ANOVA was calculated between samples (P = 0.006). Bonferroni’s multiple comparisons test on days 7 and 9 was performed to obtain adjusted P value (day 7 (P = 0.047), day 9 (P = 0.0004)). g, Multiple melanoma cells were allowed to form spheroids followed by treatment with conditioned media from aged or young fibroblasts for 48 h. The spheroids were then examined for their ability to invade in a collagen matrix. Data are represented as mean ± s.d. for each graph (b, e, f).
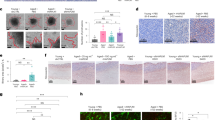
Extended Data Figure 2 sFRP2 promotes invasion and angiogenesis.
a, Conditioned media from young fibroblasts treated with either control (PBS) or rsFRP2 (200 ng ml−1) were used to pre-treat WM793 melanoma cells for 48 h. Invasion was assayed using a Boyden chamber assay over 24–72 h. Two-tailed unpaired t-test was performed (P = 0.033). b, Conditioned media from aged fibroblasts treated with sFRP2 blocking antibody (15 μg ml−1) for 72 h were used to pre-treat WM793 melanoma cells for 48 h. The invasion of melanoma cells was assessed in a Boyden chamber assay for 24–72 h. Two-tailed unpaired t-test was performed (P = 0.035). c, d, Young mice (8 weeks, 10 per group) were injected subcutaneously with Yumm1.7 cells. After palpable tumour appeared, mice were treated with rsFRP2 (200 ng ml−1) for 30 days and examined for angiogenesis using CD31 staining. Representative images, original magnification ×400. e, f, Aged mice (52 weeks, n = 5 per group) were injected subcutaneously with Yumm1.7 cells and treated with either control (e) IgG2aκ or (f) sFRP2 blocking antibody (1 mg kg−1) for 3 weeks. Tumours were examined for angiogenesis by CD31 staining. Representative images, original magnification ×400. Data are represented as mean ± s.d. for each graph (a, b).
Extended Data Figure 3 Treatment of aged tumour-bearing mice with an α-sfrp2 antibody results in a lethal inflammation.
Cytokine analysis of lungs in aged tumour-bearing mice (52 weeks, n = 5 per group) treated with IgG2aκ or α-sFRP2 antibody (1 mg kg−1, once a week for 3 weeks). RT–PCR demonstrates a difference in the lungs of mice treated with IgG2aκ or α-sFRP2 in cytokines (a) CCL5, (b) IL4, (c) IL10, (d) CXCL9, (e) IFNγ, (f) and IL2. Early response inflammatory genes (g) TNFα and (h) IL6 were no longer significantly altered. Estimate of variance was performed for all genes. For all cytokines, an two-tailed unpaired t-test was performed; *P < 0.05, **P < 0.02. Data are represented as mean ± s.d. for each graph.
Extended Data Figure 4 β-Catenin loss in the aged microenvironment.
a, β-Catenin expression in normal human skin from young and aged donors, with a focus on the fibroblast population (zoom). β-Catenin nuclear translocation in melanoma cells treated with conditioned media from aged compared with young fibroblasts as measured by (b) Western analysis and (c) a TOPFLASH assay. Two-tailed unpaired t-test was performed to indicate statistical significance between treatment with young and aged media (P = 0.023). Data are represented as mean ± s.d. (c). d, Immunofluorescent analysis of β-catenin in melanoma cells treated with media from young fibroblasts in which the β-catenin is knocked down.
Extended Data Figure 5 Increase in oxidative stress in the aged microenvironment.
a, APE1 expression in normal human skin as measured by immunohistochemistry. Slides were scored for intensity of stain (3, highest; 0, lowest; <35 years, n = 12; >55 years, n = 7). Representative images, original magnification ×400 (left) and ×200 (right). Unpaired t-test using rank sum (Mann–Whitney) revealed statistical significance (P = 0.009). Western analysis of (b) SOD3 and (c) PRDX6 levels in conditioned media from young and aged fibroblasts. d, Immunofluorescent analysis of 8-oxo-dG in normal young and aged skin stained for oxidative stress marker 8-oxo-dG (red), smooth muscle actin (green), and DAPI (blue). e, ROS activity in melanoma cells with APE1 knockdown, after exposure to aged media. ANOVA was performed for each cell line treatment (FS13 (P = 0.0006); FS14 (P = 0.004)). For FS13, a two-tailed unpaired t-test indicated significance (P < 0.01) for each shAPE1 cell line compared with control cells. For FS14, a two-tailed unpaired t-test indicated significance (P < 0.05) for each shAPE1 cell line compared with control cells. Data are represented as mean ± s.d. for each graph (a, e).
Extended Data Figure 6 Gene expression analysis of melanoma cells exposed to aged fibroblasts reveals increases in DNA damage.
a, γH2AX expression was analysed in melanoma cells exposed to H2O2 using immunofluorescence. b, Microarray analysis of the gene expression profiles of melanoma cells exposed to young/middle and aged fibroblasts identified 63 genes commonly increased in three melanoma cell lines cultured with aged versus young fibroblasts. c, Thirty-three genes involved in DNA damage response were significantly altered because of effects of ageing microenvironment in three different melanoma cell lines. Colour scale indicates expression levels relative to aged group. Y, young; M, middle; A, aged.
Extended Data Figure 7 DNA damage response is increased in melanoma cells exposed to aged fibroblasts.
Skin reconstructs made with young or aged fibroblasts were stained with (a) anti-53BP1 or (b) γH2AX and analysed by immunofluorescence. c, FS5 melanoma cell line treated with conditioned media from young and aged fibroblasts showing DNA damage as measured by a comet assay. Two-tailed unpaired t-test with Welch’s correction was performed between young and aged treatments (P = 0.039). Data are represented as mean ± s.e.m. d, Percentage ROS activity remaining after NAC treatment of aged fibroblasts. Spearman’s correlation between dose and percentage inhibition is significant (P = 0.043, r = −0.700). Data are represented as mean ± s.d. e, Knockdown of SOD3 in young fibroblasts as analysed by western blotting. f, Young fibroblasts (2003,071-032 and AG11732) were treated with rsFRP2 (200 ng ml−1) for 72 h and this conditioned medium was used to treat melanoma cells for 48 h. Cells were assessed for DNA damage by γH2AX. g, Aged fibroblasts (AG13004 and AG11726) were treated with α-sFRP2 (15 μg ml−1) for 72 h and this conditioned medium was used to treat melanoma cells for 48 h. Cells were assessed for DNA damage by γH2AX.
Extended Data Figure 8 Analysis of sFRP2, β-catenin, MITF, 8-oxo-dG, APE1, and 53BP1 in individual patients.
a, Multiple melanoma samples from aged patients (red bars), and young patients (black bars) were compared. Bars represent average staining intensity (3, highest; 0, lowest) in all patients (n = 9 per group) for indicated proteins. b–g, Dot plots of staining intensity (0–3+) in individual patient samples for (b) sFRP2, (c) β-catenin, (d) MITF, (e) APE1, (f) 8-oxodG, and (g) 53BP1. P values for each graph obtained by Mann–Whitney tests.
Extended Data Figure 9 β-Catenin predicts for sensitivity to vemurafenib.
a, Melanoma spheroids were embedded in collagen and treated with 1 μM PLX4720 in the presence of conditioned media from either young or aged fibroblasts. After 48 h, spheroids were assessed for cell death by staining with ethidium homodimer (original magnification ×40). b, In cells intrinsically sensitive to vemurafenib in culture, β-catenin expression is increased. c, Knockdown of β-catenin in Yumm1.7 cells decreases their sensitivity to PLX4720. Spearman’s correlation between dose and percentage proliferation is significant in control cells (P < 0.0001, r = −1.000) whereas shCTNNB1 cells indicated no significant changes in curve after treatment (P = 0.948, r = 0.03). d, Young mice (8 weeks, n = 10 per group) were injected with rsFRP2 (200 ng ml−1, twice weekly) and skin was examined for β-catenin levels by immunohistochemistry. Representative images, original magnification ×200. e, Yumm1.7 tumours were injected in aged mice pre-treated with α-sFRP2 antibody (1 mg kg−1, once weekly). Mice were then administered either control or 417 mg kg−1 PLX4720-laced chow. ANOVA is significant between treatments (P < 0.0001). Two-tailed unpaired t-test using rank sum (Mann–Whitney) was performed on tumour volumes on day 25 (1 week after treatment). Results were significant in sFRP2 treatment (P = 0.036) and insignificant in IgG2aκ treatment (P = 0.057). f, Patient samples show a continuum of decreased response in relation to age, Spearman’s correlation between percentage response and age is significant (r = 0.243, P = 0.035). Data are represented as mean ± s.d. for each graph (c, e).
Supplementary information
Supplementary Figure
This file contains the raw data for Figures 2a, 2g, 3a, 3e, 4f, 4g, 5b and Extended Data Figures 6b, 6c, 8e, 10b, 10c. (PDF 7726 kb)
Supplementary Tables
This file is a compilation of tables outlining the antibodies, vectors and primers used, and the sources from which they came. Extended statistics for patient data, as well as patient information are also included. (PDF 512 kb)
Rights and permissions
About this article
Cite this article
Kaur, A., Webster, M., Marchbank, K. et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 532, 250–254 (2016). https://doi.org/10.1038/nature17392
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17392