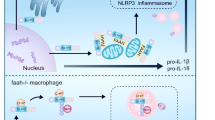
Abstract
Inflammasomes are intracellular protein complexes that drive the activation of inflammatory caspases1. So far, four inflammasomes involving NLRP1, NLRP3, NLRC4 and AIM2 have been described that recruit the common adaptor protein ASC to activate caspase-1, leading to the secretion of mature IL-1β and IL-18 proteins2,3. The NLRP3 inflammasome has been implicated in the pathogenesis of several acquired inflammatory diseases4,5 as well as cryopyrin-associated periodic fever syndromes (CAPS) caused by inherited NLRP3 mutations6,7. Potassium efflux is a common step that is essential for NLRP3 inflammasome activation induced by many stimuli8,9. Despite extensive investigation, the molecular mechanism leading to NLRP3 activation in response to potassium efflux remains unknown. Here we report the identification of NEK7, a member of the family of mammalian NIMA-related kinases (NEK proteins)10, as an NLRP3-binding protein that acts downstream of potassium efflux to regulate NLRP3 oligomerization and activation. In the absence of NEK7, caspase-1 activation and IL-1β release were abrogated in response to signals that activate NLRP3, but not NLRC4 or AIM2 inflammasomes. NLRP3-activating stimuli promoted the NLRP3–NEK7 interaction in a process that was dependent on potassium efflux. NLRP3 associated with the catalytic domain of NEK7, but the catalytic activity of NEK7 was shown to be dispensable for activation of the NLRP3 inflammasome. Activated macrophages formed a high-molecular-mass NLRP3–NEK7 complex, which, along with ASC oligomerization and ASC speck formation, was abrogated in the absence of NEK7. NEK7 was required for macrophages containing the CAPS-associated NLRP3(R258W) activating mutation to activate caspase-1. Mouse chimaeras reconstituted with wild-type, Nek7−/− or Nlrp3−/− haematopoietic cells showed that NEK7 was required for NLRP3 inflammasome activation in vivo. These studies demonstrate that NEK7 is an essential protein that acts downstream of potassium efflux to mediate NLRP3 inflammasome assembly and activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002)
Rathinam, V. A., Vanaja, S. K. & Fitzgerald, K. A. Regulation of inflammasome signaling. Nature Immunol. 13, 333–342 (2012)
Guo, H., Callaway, J. B. & Ting, J. P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature Med. 21, 677–687 (2015)
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009)
Davis, B. K., Wen, H. & Ting, J. P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735 (2011)
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nature Genet. 29, 301–305 (2001)
Feldmann, J. et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71, 198–203 (2002)
Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013)
Pétrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007)
Fry, A. M., O'Regan, L., Sabir, S. R. & Bayliss, R. Cell cycle regulation by the NEK family of protein kinases. J. Cell Sci. 125, 4423–4433 (2012)
Richards, M. W. et al. An autoinhibitory tyrosine motif in the cell-cycle-regulated Nek7 kinase is released through binding of Nek9. Mol. Cell 36, 560–570 (2009)
Yissachar, N., Salem, H., Tennenbaum, T. & Motro, B. Nek7 kinase is enriched at the centrosome, and is required for proper spindle assembly and mitotic progression. FEBS Lett. 580, 6489–6495 (2006)
O'Regan, L. & Fry, A. M. The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis. Mol. Cell. Biol. 29, 3975–3990 (2009)
Salem, H. et al. Nek7 kinase targeting leads to early mortality, cytokinesis disturbance and polyploidy. Oncogene 29, 4046–4057 (2010)
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011)
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013)
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013)
Neven, B. et al. Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood 103, 2809–2815 (2004)
Meng, G., Zhang, F., Fuss, I., Kitani, A. & Strober, W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity 30, 860–874 (2009)
Kofoed, E. M. & Vance, R. E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 (2011)
Belham, C. et al. A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J. Biol. Chem. 278, 34897–34909 (2003)
Cohen, S., Aizer, A., Shav-Tal, Y., Yanai, A. & Motro, B. Nek7 kinase accelerates microtubule dynamic instability. Biochim. Biophys. Acta 1833, 1104–1113 (2013)
Kim, S., Lee, K. & Rhee, K. NEK7 is a centrosomal kinase critical for microtubule nucleation. Biochem. Biophys. Res. Commun. 360, 56–62 (2007)
Ito, M. et al. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nature Commun. 6, 7360 (2015)
Sutterwala, F. S. et al. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 (2006)
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006)
Zaki, M. H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010)
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature Immunol. 11, 136–140 (2010)
Schmid-Burgk, J. L. et al. A genome-wide CRISPR screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J. Biol. Chem. 291, 103–109 (2015)
Shi, H. et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nature Immunol. http://dx.doi.org/10.1038/ni.3333 (2015)
Kanneganti, T. D. et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 (2006)
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998)
He, Y., Franchi, L. & Nunez, G. TLR agonists stimulate Nlrp3-dependent IL-1beta production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J. Immunol. 190, 334–339 (2013)
Blasi, E. et al. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature 318, 667–670 (1985)
Swamy, M., Siegers, G. M., Minguet, S., Wollscheid, B. & Schamel, W. W. Blue native polyacrylamide gel electrophoresis (BN-PAGE) for the identification and analysis of multiprotein complexes. Sci. STKE 2006, pl4 (2006)
Acknowledgements
We thank R. Muñoz-Planillo for potassium efflux assays, S. Varadarajan for plasmid construction, L. Burmeister for animal husbandry, J. Whitfield for ELISA assays, and V. Basrur for mass spectrometry analysis. Y.H. was supported by NIH training grant T32HL007517. M.Y.Z. was supported by NIH training grants T32HL007517 and T32DK094775. D.Y. was supported by the State Scholarship Fund from China Scholarship Council (no. 201306740018). This work was supported by NIH grants R01AI063331 and R01DK091191 to G.N., research funds by the Israel Science Foundation (grant no. 768/11) to B.M., and funds to the Michigan Comprehensive Cancer Center Immunology Monitoring Core from the University of Michigan’s Cancer Center Support Grant.
Author information
Authors and Affiliations
Contributions
Y.H. and G.N. designed the research and wrote the manuscript. Y.H. conducted the experiments and analysed data with the help from M.Y.Z. and D.Y., and B.M. generated and provided critical material. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 NEK7 interacts with NLRP3 upstream of ASC and caspase-1.
a, Immortalized Nlrp3−/− macrophages were infected with lentiviruses containing pHIV vector (Vector) or pHIV-NLRP3-SFP (NLRP3-SFP). Transduced macrophages were sorted by flow cytometry. Cells were treated as indicated. Supernatants and cell lysates were collected and analysed by immunoblotting with indicated antibodies. b, Immunoblots showing that NEK7, but not NEK6 or NEK9, interacts with NLRP3 in macrophages. NLRP3-associated proteins were pulled down from reconstituted Nlrp3−/− macrophages by streptavidin beads, and analysed by immunoblotting with indicated antibodies. c, ATP stimulation, but not LPS priming, greatly enhances the NEK7–NLRP3 interaction. d, LPS-primed BMDMs from wild-type, Asc−/− and Casp1−/− Casp11−/− were left untreated or treated with 5 mM ATP for 30 min. NLRP3 protein complexes were immunoprecipitated with anti-NLRP3 antibody and analysed by immunoblotting. Data are representative of three (a, b) or two (c, d) independent experiments. See Supplementary Figs 2 and 3 for gel source data.
Extended Data Figure 2 NEK7 deficiency does not affect TNF-α release, but abrogates NLRP3 activation induced by cytosolic LPS in macrophages.
a, TNF-α secretion from LPS-primed BMDMs treated with ATP, nigericin, gramicidin, poly(dA:dT) or Salmonella. b, TNF-α secretion in LPS-primed BMDMs treated with LLOMe, silica, Alum, MSU, CPPD or nano-SiO2. Mock represents macrophages primed with LPS without further stimulation. The amounts of TNF-α release in the absence of LPS priming were below 50 pg ml−1 in the supernatants. c–e, BMDMs from C57BL/6, Casp11−/−, Nek7−/− (fetal liver chimaeras) and Nlrp3−/− mice were primed with LPS for 4 h before transfection with or without LPS with DOTAP. Four hours after transfection, caspase-1 activation (c), IL-1β release (d) and cytotoxicity (e) were analysed. Graphs show the mean and s.d. of triplicate wells, and are representative of three independent experiments. See Supplementary Fig. 3 for gel source data.
Extended Data Figure 3 NEK7 is required for activation of the NLRP3 inflammasome in macrophages.
a–c, Defective NLRP3 inflammasome in NEK7-deficient macrophages. Wild-type and NEK7-deficient macrophages generated by CRISPR-Cas9 genome editing were primed with LPS, followed by stimulation with 5 μM nigericin (1 h), 5 mM ATP (30 min) or 200 ng ml−1 Alum (6 h). Cell lysates were collected and analysed for caspase-1 activation by immunoblotting (a). IL-1β (b) and TNF-α (c) release were analysed by ELISA. ELISA data (b, c) show the mean and s.d. of triplicate wells. The amounts of TNF-α release in the absence of LPS priming were below 50 pg ml−1 in the supernatants. Data are representative of three independent experiments. See Supplementary Fig. 3 for gel source data.
Extended Data Figure 4 NEK7 is critical for activation of the NLRP3 inflammasome in primary macrophages.
a, NEK7 is dispensable for NLRP3 induction and expression of the NLRP3 inflammasome components ASC and caspase-1. BMDMs were treated with control shRNA or Nek7 shRNAs and selected by culture in 3 μg ml−1 puromycin. Puromycin-resistant macrophages were left unstimulated or stimulated with LPS for 4 h. Cell lysates were collected and analysed by immunoblotting. Actin is shown as a loading control. b, c, NEK7 depletion inhibits activation of the NLRP3 inflammasome. BMDMs treated with control shRNA or Nek7 shRNAs were primed with LPS, followed by stimulation with 5 mM ATP (30 min), 5 μM nigericin (1 h) or 500 ng ml−1 silica (4 h). Supernatants and cell lysates were collected after stimulation and analysed by immunoblotting for caspase-1 activation (b). IL-1β (c) and TNF-α (d) release were analysed by ELISA. ELISA data (c, d) show the mean and s.d. of triplicate wells. The amounts of TNF-α release in the absence of LPS priming were below 50 pg ml−1 in the supernatants. Data are representative of three independent experiments. See Supplementary Fig. 3 for gel source data.
Extended Data Figure 5 NEK7 is critical for NLRP3-mediated ASC oligomerization and speck formation in macrophages.
a, b, Representative confocal immunofluorescence images (a) and quantification of endogenous ASC specks (arrows) (b) in macrophages treated with control shRNA or Nek7 shRNA. Macrophages were primed with LPS and then stimulated with ATP, nigericin, poly(dA:dT) or Salmonella. After stimulation, cells were fixed and stained for ASC (green). DAPI was used for staining nuclear DNA (blue). Data shown represent results from three combined independent experiments in which more than 300 cells were counted in each experiment. Error bars indicate s.d. Original magnifications, ×400. c, Immunoblots showing ASC oligomerization in control-shRNA-treated or Nek7-shRNA-treated macrophages. LPS-primed macrophages were treated as indicated. Cell lysates (Triton X-100 soluble) and BS3-crosslinked pellets (Triton X-100 insoluble) were analysed by immunoblotting. Mock represents macrophages primed with LPS without further stimulations. Results are representative of two independent experiments. *P < 0.05 (two-tailed Student’s t-test). See Supplementary Fig. 3 for gel source data.
Extended Data Figure 6 NEK7 kinase activity is dispensable for activation of the NLRP3 inflammasome in macrophages.
a–c, Immortalized NEK7-deficient macrophages were infected with lentivirus containing empty vector or vector expressing wild-type or indicated mutant NEK7. Transduced macrophages were sorted by flow cytometry using GFP as a marker. Macrophages were plated and stimulated with 5 μM nigericin for 1 h after LPS priming. Cell lysates were collected and analysed for caspase-1 activation by immunoblotting (a). IL-1β (b) and TNF-α (c) release were analysed by ELISA. ELISA data (b, c) show the mean and s.d. of triplicate wells. Data are representative of three independent experiments. See Supplementary Fig. 3 for gel source data.
Extended Data Figure 7 NEK6 and NEK9 are dispensable for activation of the NLRP3 inflammasome in macrophages.
a–c, BMDMs were treated with control shRNA, Nek7 shRNA, Nek6 shRNA or Nek9 shRNA and selected by culture with 3 μg ml−1 puromycin. Puromycin-resistant cells were plated and primed with LPS, followed by stimulation with 5 mM ATP for 30 min. Cell lysates were collected and analysed for caspase-1 activation by immunoblotting (a). IL-1β (b) and TNF-α (c) release were analysed by ELISA. d, Representative confocal immunofluorescence images (d) and quantification of endogenous ASC specks (arrows) (e) in macrophages. DAPI was used for staining nuclear DNA (blue). Quantification of ASC speck was from three combined independent experiments in which more than 300 cells were counted in each experiment. Error bars indicate s.d. Scale bars, 10 μm. ELISA data (b, c) show the mean and s.d. of triplicate wells. Data are representative of three independent experiments. *P < 0.05 (two-tailed Student’s t-test). See Supplementary Fig. 3 for gel source data.
Extended Data Figure 8 Microtubule depolymerization does not inhibit the activation of the NLRP3 inflammasome and the NEK7–NLRP3 interaction in macrophages.
a–e, LPS-primed BMDMs were pretreated with vehicle control (DMSO), nocodazole (1, 10 or 50 μM) or colchicine (1, 10 or 50 μM) for 1 h before stimulation with 5 μM nigericin. Cell lysates were collected and analysed for caspase-1 activation by immunoblotting (a). IL-1β (b) and TNF-α (c) release were analysed by ELISA. Representative confocal immunofluorescence images (d) and quantification (e) of endogenous ASC specks (arrows) in macrophages treated as indicated. Microtubules were stained with anti-α/β tubulin antibody (red). DAPI was used for staining nuclear DNA (blue). Data shown in e represent results from three combined independent experiments in which more than 300 cells were counted in each experiment. Error bars indicate s.d. Scale bars, 10 μm. f, The NEK7–NLRP3 interaction in untreated and treated macrophages was analysed by immunoprecipitation and immunoblotting. ELISA data (b, c) show the mean and s.d. of triplicate wells. Data are representative of three independent experiments. *P < 0.05 (two-tailed Student’s t-test). See Supplementary Fig. 3 for gel source data.
Extended Data Figure 9 Inhibition of BTK does not inhibit the NEK7–NLRP3 interaction in macrophages.
a, b, Reconstituted immortalized Nlrp3−/− macrophages were primed with LPS for 4 h. Cells were left untreated or treated with indicated concentrations of BTK inhibitor LFM-A13 for 30 min before ATP stimulation. Cell lysates were collected and analysed for caspase-1 activation by immunoblotting (a). Cell lysates were collected and subjected to pull-down with streptavidin beads, and the precipitated protein complex was analysed by immunoblotting with indicated antibodies (b). Agarose beads were used as a control. Actin is shown as a loading control. Data are representative of two independent experiments. See Supplementary Fig. 3 for gel source data.
Extended Data Figure 10 NEK7-deficiency does not inhibit potassium efflux induced by NLRP3 stimuli in macrophages.
LPS-primed Nek7+/+ and Nek7−/− BMDMs were stimulated as indicated. The intracellular potassium in each condition was analysed. Mock represents macrophages primed with LPS without further stimulation. Graphs show the mean and s.d. of four technical replicates and are representative of two independent experiments.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-3, which contain gel source data. (PDF 1460 kb)
Rights and permissions
About this article
Cite this article
He, Y., Zeng, M., Yang, D. et al. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016). https://doi.org/10.1038/nature16959
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature16959