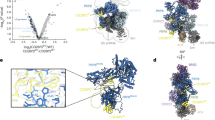
Abstract
U4/U6.U5 tri-snRNP is a 1.5-megadalton pre-assembled spliceosomal complex comprising U5 small nuclear RNA (snRNA), extensively base-paired U4/U6 snRNAs and more than 30 proteins, including the key components Prp8, Brr2 and Snu114. The tri-snRNP combines with a precursor messenger RNA substrate bound to U1 and U2 small nuclear ribonucleoprotein particles (snRNPs), and transforms into a catalytically active spliceosome after extensive compositional and conformational changes triggered by unwinding of the U4 and U6 (U4/U6) snRNAs. Here we use cryo-electron microscopy single-particle reconstruction of Saccharomyces cerevisiae tri-snRNP at 5.9 Å resolution to reveal the essentially complete organization of its RNA and protein components. The single-stranded region of U4 snRNA between its 3′ stem–loop and the U4/U6 snRNA stem I is loaded into the Brr2 helicase active site ready for unwinding. Snu114 and the amino-terminal domain of Prp8 position U5 snRNA to insert its loop I, which aligns the exons for splicing, into the Prp8 active site cavity. The structure provides crucial insights into the activation process and the active site of the spliceosome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Will, C. L. & Lührmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 3, a003707 (2011)
Chan, S. P. & Cheng, S. C. The Prp19-associated complex is required for specifying interactions of U5 and U6 with pre-mRNA during spliceosome activation. J. Biol. Chem. 280, 31190–31199 (2005)
Fabrizio, P. et al. The evolutionarily conserved core design of the catalytic activation step of the yeast spliceosome. Mol. Cell 36, 593–608 (2009)
Fica, S. M. et al. RNA catalyses nuclear pre-mRNA splicing. Nature 503, 229–234 (2013)
Newman, A. J. & Norman, C. U5 snRNA interacts with exon sequences at 5′ and 3′ splice sites. Cell 68, 743–754 (1992)
Sontheimer, E. J. & Steitz, J. A. The U5 and U6 small nuclear RNAs as active site components of the spliceosome. Science 262, 1989–1996 (1993)
Stevens, S. W. et al. Biochemical and genetic analyses of the U5, U6, and U4/U6.U5 small nuclear ribonucleoproteins from Saccharomyces cerevisiae. RNA 7, 1543–1553 (2001)
Gottschalk, A. et al. Identification by mass spectrometry and functional analysis of novel proteins of the yeast [U4/U6.U5] tri-snRNP. EMBO J. 18, 4535–4548 (1999)
Turner, I. A., Norman, C. M., Churcher, M. J. & Newman, A. J. Dissection of Prp8 protein defines multiple interactions with crucial RNA sequences in the catalytic core of the spliceosome. RNA 12, 375–386 (2006)
Galej, W. P., Oubridge, C., Newman, A. J. & Nagai, K. Crystal structure of Prp8 reveals active site cavity of the spliceosome. Nature 493, 638–643 (2013)
Fabrizio. P., Laggerbauer, B., Lauber, J., Lane, W. S. & Lührmann, R. An evolutionarily conserved U5 snRNP-specific protein is a GTP-binding factor closely related to the ribosomal translocase EF-2. EMBO J. 16, 4092–4106 (1997)
Small, E. C., Leggett, S. R., Winans, A. A. & Staley, J. P. The EF-G-like GTPase Snu114p regulates spliceosome dynamics mediated by Brr2p, a DExD/H box ATPase. Mol. Cell 23, 389–399 (2006)
Bartels. C., Urlaub, H., Lührmann, R. & Fabrizio P. Mutagenesis suggests several roles of Snu114p in pre-mRNA splicing. J. Biol. Chem. 278, 28324–28334 (2003)
Raghunathan, P. L. & Guthrie, C. RNA unwinding in U4/U6 snRNPs requires ATP hydrolysis and the DEIH-box splicing factor Brr2. Curr. Biol. 8, 847–855 (1998)
Laggerbauer, B., Achsel, T. & Lührmann, R. The human U5–200kD DEXH-box protein unwinds U4/U6 RNA duplices in vitro. Proc. Natl Acad. Sci. USA 95, 4188–4192 (1998)
Liu, S., Rauhut, R., Vornlocher, H. P. & Lührmann, R. The network of protein-protein interactions within the human U4/U6.U5 tri-snRNP. RNA 12, 1418–1430 (2006)
van Nues, R. W. & Beggs, J. D. Functional contacts with a range of splicing proteins suggest a central role for Brr2p in the dynamic control of the order of events in spliceosomes of Saccharomyces cerevisiae. Genetics 157, 1451–1467 (2001)
Sander, B. et al. Organization of core spliceosomal components U5 snRNA loop I and U4/U6 Di-snRNP within U4/U6.U5 Tri-snRNP as revealed by electron cryomicroscopy. Mol. Cell 24, 267–278 (2006)
Häcker, I. et al. Localization of Prp8, Brr2, Snu114 and U4/U6 proteins in the yeast tri-snRNP by electron microscopy. Nature Struct. Mol. Biol. 15, 1206–1212 (2008)
McMullan, G. et al. Experimental observation of the improvement in MTF from backthinning a CMOS direct electron detector. Ultramicroscopy 109, 1144–1147 (2009)
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nature Methods 10, 584–590 (2013)
Scheres, S. H. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 415, 406–418 (2012)
Bai, X. C., McMullan, G. & Scheres, S. H. How cryo-EM is revolutionizing structural biology. Trends Biochem. Sci. 40, 49–57 (2015)
Scheres, S. H. Beam-induced motion correction for sub-megadalton cryo-EM particles. Elife 3, e03665 (2014)
Scheres, S. H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nature Methods 9, 853–854 (2012)
Jørgensen, R. et al. Two crystal structures demonstrate large conformational changes in the eukaryotic ribosomal translocase. Nature Struct. Biol. 10, 379–385 (2003)
Grainger, R. J., Barrass, J. D., Jacquier, A., Rain, J. C. & Beggs, J. D. Physical and genetic interactions of yeast Cwc21p, an ortholog of human SRm300/SRRM2, suggest a role at the catalytic center of the spliceosome. RNA 15, 2161–2173 (2009)
Dix, I., Russell, C. S., O'Keefe, R. T., Newman, A. J. & Beggs, J. D. Protein-RNA interactions in the U5 snRNP of Saccharomyces cerevisiae. RNA 4, 1675–1686 (1998)
Reuter, K., Nottrott, S., Fabrizio, P., Lührmann, R. & Ficner, R. Identification, characterization and crystal structure analysis of the human spliceosomal U5 snRNP-specific 15 kD protein. J. Mol. Biol. 294, 515–525 (1999)
Maeder, C., Kutach, A. K. & Guthrie, C. ATP-dependent unwinding of U4/U6 snRNAs by the Brr2 helicase requires the C terminus of Prp8. Nature Struct. Mol. Biol. 16, 42–48 (2009)
Nguyen, T. H. D. et al. Structural basis of Brr2-Prp8 interactions and implications for U5 snRNP biogenesis and the spliceosome active site. Structure 21, 910–919 (2013)
Mozaffari-Jovin, S. et al. Inhibition of RNA helicase Brr2 by the C-terminal tail of the spliceosomal protein Prp8. Science 341, 80–84 (2013)
Liu, S. et al. Binding of the human Prp31 Nop domain to a composite RNA-protein platform in U4 snRNP. Science 316, 115–120 (2007)
Schultz, A., Nottrott, S., Hartmuth, K. & Lührmann, R. RNA structural requirements for the association of the spliceosomal hPrp31 protein with the U4 and U4atac small nuclear ribonucleoproteins. J. Biol. Chem. 281, 28278–28286 (2006)
Ayadi, L. et al. Functional and structural characterization of the Prp3 binding domain of the yeast Prp4 splicing factor. J. Mol. Biol. 284, 673–687 (1998)
Korneta, I., Magnus, M. & Bujnicki, J. M. Structural bioinformatics of the human spliceosomal proteome. Nucleic Acids Res. 40, 7046–7065 (2012)
Nottrott, S., Urlaub, H. & Lührmann, R. Hierarchical, clustered protein interactions with U4/U6 snRNA: a biochemical role for U4/U6 proteins. EMBO J. 21, 5527–5538 (2002)
Galisson, F. & Legrain, P. The biochemical defects of prp4–1 and prp6–1 yeast splicing mutants reveal that the PRP6 protein is required for the accumulation of the [U4/U6.U5] tri-snRNP. Nucleic Acids Res. 21, 1555–1562 (1993)
Makarov, E. M., Makarova, O. V., Achsel, T. & Lührmann, R. The human homologue of the yeast splicing factor prp6p contains multiple TPR elements and is stably associated with the U5 snRNP via protein-protein interactions. J. Mol. Biol. 298, 567–575 (2000)
Boon, K. L. et al. Prp8 mutations that cause human retinitis pigmentosa lead to a U5 snRNP maturation defect in yeast. Nature Struct. Mol. Biol. 14, 1077–1083 (2007)
Mozaffari-Jovin, S. et al. The Prp8 RNase H-like domain inhibits Brr2-mediated U4/U6 snRNA unwinding by blocking Brr2 loading onto the U4 snRNA. Genes Dev. 26, 2422–2434 (2012)
Hahn, D., Kudla, G., Tollervey, D. & Beggs, J. D. Brr2p-mediated conformational rearrangements in the spliceosome during activation and substrate repositioning. Genes Dev. 26, 2408–2421 (2012)
Büttner, K., Nehring, S. & Hofner, K. P. Structural basis for DNA duplex separation by a superfamily‐2 helicase. Nature Struct. Mol. Biol. 14, 647–652 (2007)
Tourigny, D. S., Fernández, I. S., Kelley, A. C. & Ramakrishnan, V. Elongation factor G bound to the ribosome in an intermediate state of translocation. Science 340, 1235490 (2013)
Lin. J, Gagnon, M. G., Bulkley, D. & Steitz, T. A. Conformational changes of elongation factor G on the ribosome during tRNA translocation. Cell 160, 219–227 (2015)
Kuhn, A. N. & Brow, D. A. Suppressors of a cold-sensitive mutation in yeast U4 RNA define five domains in the splicing factor Prp8 that influence spliceosome activation. Genetics 155, 1667–1682 (2000)
Li, Z. & Brow, D. A. A spontaneous duplication in U6 spliceosomal RNA uncouples the early and late functions of the ACAGA element in vivo. RNA 2, 879–894 (1996)
Toor, N., Keating, K. S., Taylor, S. D. & Pyle, A. M. Crystal structure of a self-spliced group II intron. Science 320, 77–82 (2008)
Fica, S. M., Mefford, M. A., Piccirilli, J. A. & Staley, J. P. Evidence for a group II intron-like catalytic triplex in the spliceosome. Nature Struct. Mol. Biol. 21, 464–471 (2013)
Sharp, P. A. Five easy pieces. Science 254, 663 (1991)
Schreieck, A. et al. RNA polymerase II termination involves C-terminal domain tyrosine dephosphorylation by CPF subunit Glc7. Nature Struct. Mol. Biol. 21, 175–179 (2014)
Mindell, J. A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003)
Elmlund, H., Elmlund, D. & Bengio, S. PRIME: probabilistic initial 3D model generation for single-particle cryo-electron microscopy. Structure 21, 1299–1306 (2013)
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nature Methods 11, 63–65 (2014)
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013)
Scheres, S. H. W., Nuñez-Ramirez, R., Sorzano, C. O. S., Carazo, J. M. & Marabini, R. Image processing for electron microscopy single-particle analysis using Xmipp. Nature Protocols 3, 977–990 (2008)
Tang, G. et al. EMAN2: an extensive image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007)
Goddard, T. D., Huang, C. C. & Ferrin, T. E. Visualizing density maps with UCSF Chimera. J. Struct. Biol. 157, 281–287 (2007)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010)
Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9, 40 (2008)
Malmström, L. et al. Superfamily assignments for the yeast proteome through integration of structure prediction with the gene ontology. PLoS Biol. 5, e76 (2007)
Wu, X. H., Chen, R. C., Gao, Y. & Wu, Y. D. The effect of Asp-His-Ser/Thr-Trp tetrad on the thermostability of WD40-repeat proteins. Biochemistry 49, 10237–10245 (2010)
Rother, M. et al. ModeRNA server: an online tool for modeling RNA 3D structures. Bioinformatics 27, 2441–2442 (2011)
Dobbyn, H. C. et al. Analysis of pre-mRNA and pre-rRNA processing factor Snu13p structure and mutants. Biochem. Biophys. Res. Commun. 360, 857–862 (2007)
Leung, A. K., Nagai, K. & Li, J. Structure of the spliceosomal U4 snRNP core domain and its implication for snRNP biogenesis. Nature 473, 536–539 (2011)
Chanfreau, G., Elela, S. A., Ares, M., Jr & Guthrie, C. Alternative 3′-end processing of U5 snRNA by RNase III. Genes Dev. 11, 2741–2751 (1997)
Zhou, L. et al. Crystal structures of the Lsm complex bound to the 3′end sequence of U6 small nuclear RNA. Nature 506, 116–120 (2014)
Query, C. C. & Konarska, M. M. Suppression of multiple substrate mutations by spliceosomal prp8 alleles suggests functional correlations with ribosomal ambiguity mutants. Mol. Cell 14, 343–354 (2004)
Umen, J. G. & Guthrie, C. Mutagenesis of the yeast gene PRP8 reveals domains governing the specificity and fidelity of 3′ splice site selection. Genetics 143, 723–739 (1996)
Liu, L., Query, C. C. & Konarska, M. M. Opposing classes of prp8 alleles modulate the transition between the catalytic steps of pre-mRNA splicing. Nature Struct. Mol. Biol. 14, 519–526 (2007)
Dagher, S. F. & Fu, X. D. Evidence for a role of Sky1p-mediated phosphorylation in 3′ splice site recognition involving both Prp8 and Prp17/Slu4. RNA 7, 1284–1297 (2001)
Ben-Yehuda, S. et al. Extensive genetic interactions between PRP8 and PRP17/CDC40, two yeast genes involved in pre-mRNA splicing and cell cycle progression. Genetics 154, 61–71 (2000)
Collins, C. A. & Guthrie, C. Allele-specific genetic interactions between Prp8 and RNA active site residues suggest a function for Prp8 at the catalytic core of the spliceosome. Genes Dev. 13, 1970–1982 (1999)
Siatecka, M., Reyes, J. L. & Konarska, M. M. Functional interactions of Prp8 with both splice sites at the spliceosomal catalytic center. Genes Dev. 13, 1983–1993 (1999)
Kuhn, A. N., Li, Z. & Brow, D. A. Splicing factor Prp8 governs U4/U6 RNA unwinding during activation of the spliceosome. Mol. Cell 3, 65–75 (1999)
Acknowledgements
We thank S. Chen, G. McMullan, J. Grimmett and T. Darling for smooth running of the EM and computing facilities; P. da Fonseca, N. Unwin, I. Sanchez Fernandez, A. Amunts, P. Emsley, G. Murshudov and A. Brown for advice; A. Easter and L. Passmore for reagents; M. Skehel for mass spectrometry; and J. Li, Y. Kondo and the members of the spliceosome group for help and advice throughout the project. We are grateful to R. Henderson, D. Barford, S. Fica, P.-C. Lin and L. Strittmatter for critical reading of the manuscript. We thank V. Ramakrishnan, J. Löwe and R. Henderson for their continuing support and encouragements. T.H.D.N. was supported in part by a Herchel Smith Research Studentship. X.-c.B. was supported by a European Union Marie Curie Fellowship. The project was supported by the Medical Research Council (MC_U105184330 to K.N. and MC_UP_A025_1013 to S.H.W.S.).
Author information
Authors and Affiliations
Contributions
T.H.D.N. developed the purification procedure for yeast tri-snRNP, prepared EM grids, collected all EM images, processed data, calculated the maps and built and fitted most of the components into the map. W.P.G. built most of the unknown components and made essential contributions to sequence analysis, homology modelling and model fitting. X.-c.B. helped T.H.D.N. with image processing and map calculation. C.G.S. guided T.H.D.N. with EM sample preparation and data collection. A.J.N. produced the Brr2 TAPS-tagged strain and contributed to the project through his knowledge of yeast spliceosome. T.H.D.N. and W.P.G prepared all illustrations. T.H.D.N prepared the video. S.H.W.S. carried out multi-body refinement and oversaw the EM analysis. K.N. initiated and orchestrated the project. T.H.D.N., W.P.G., A.J.N. and K.N. interpreted the results and wrote the paper with crucial contribution from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
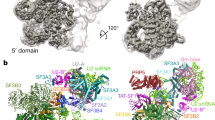
Extended Data Figure 1 U4/U6.U5 tri-snRNP sample used for this study.
a, Coomassie-blue-stained SDS–PAGE gel showing protein composition of the purified tri-snRNP. U5-, U4/U6- and tri-snRNP-specific proteins are labelled in blue, red and teal, respectively. Sm proteins present in both U5 and U4/U6 are in black. b, Toluidine-blue-stained denaturing acrylamide (9%) gel showing RNA compositions. c, Electron cryo-micrograph of tri-snRNP where the carbon-coated grid was discharged in N-amylamine. d, e, Reference-free two-dimensional class averages of a data set collected on a grid discharged in air and N-amylamine, respectively.
Extended Data Figure 2 Classification and refinement procedures used in this study.
A total of 367,327 particles were subjected to reference-free 2D classification. A subset of 347,241 particles from good 2D classes was selected for 3D classification using an initial model obtained from SIMPLE-PRIME53, which was low-pass filtered to 60 Å. The data were divided into four 3D classes, two of which (a total of 179,079 particles) showed better features and were combined for refinement. This resulted in a 7.6 Å reconstruction. To further improve the reconstruction, these particles were subjected to beam-induced motion correction (particle polishing)24. Refinement of these polished particles with a soft mask around the rigid part of the map (as indicated by the red envelope) yielded a 5.9 Å reconstruction while refinement with a mask around the whole map yielded a 6.4 Å reconstruction. The polished particles were also subject to further 3D classification with a finer angular sampling of 1.8°. The most populated class (47,674 particles), which also has the best rotational accuracy, was refined with a soft mask around the whole density. This resulted in a 7.0 Å reconstruction. In this study, the 5.9 Å reconstruction was used for subsequent biological interpretation. All steps were performed in RELION22 unless otherwise stated.
Extended Data Figure 3 CryoEM maps and tilt-pair validation.
a, CryoEM density of the whole tri-snRNP at 5.9 Å resolution by ‘gold standard’ Fourier shell correlation (FSC) of 0.143 criterion at two different contour levels. The high contour map (gold) shows well-resolved densities for protein and RNA helices and flat densities for β-sheets. The low contour map (silver) shows densities for the more flexible head and arm. The map was sharpened by a B-factor of −214 Å2 and low-pass filtered to 5.9 Å as determined by RELION. b, The unsharpened full map of tri-snRNP. c, The map resulting from multi-body refinement, in which tri-snRNP is divided into four parts: the head, body, arm and foot. This resulted in better density for the arm domain (indicated by red circles), which is at 20 Å resolution. d, Tilt-pair validation plot for tri-snRNP. This was obtained from 1,196 particles from 32 micrograph pairs, imaged at 0° and 10° tilt angles. The position of each dot represents the direction and the amount of tilting for a particle pair in polar coordinates. Blue dots correspond to in-plane tilt transformations; red and purple dots correspond to out-of-plane tilt transformations. Blue dots cluster in the same region of the plot at a tilt angle of approximately 10° as indicated by the red circle.
Extended Data Figure 4 Resolution estimation of tri-snRNP map.
a, Local resolution of the tri-snRNP map estimated by ResMap using the colour scheme shown in panel c. b, Local resolution of the tri-snRNP map calculated by ‘gold-standard’ FSC. For each component of the map that we modelled protein/RNA components, a soft mask (with a 30-pixel soft edge) surrounding the region of interest was prepared and used for FSC calculations. Convolution effects of the masks on the FSC curves were corrected using high-resolution noise substitution55. Resolution was estimated at FSC = 0.143. Local resolution for the unmodelled region of the map (in red) was not estimated. c, Local resolution of model versus map. The map of each modelled component was extracted from the map using a soft mask (with a 5-pixel soft edge) surrounding the component. The model was converted into density by EMAN57. FSC of model versus map was calculated using Xmipp56. The map is coloured according to resolution estimates based on a FSC threshold of 0.25. The lower resolution estimates from the FSC of model versus map compared to the estimates from ResMap and the gold-standard FSCs are explained by the nature of our models. Because of the limited resolution of our map, we did not perform full atomic refinement, but placed known crystal structures and homology models as rigid bodies in the map. d, Gold-standard FSC curves for the whole tri-snRNP map and some of its components calculated as described in b. e, FSC curves of model versus map for the whole model and some of the components. f, The full tri-snRNP map in which portions of the structure produced from crystal structures, homology modelling and de novo building or unmodelled are coloured as indicated.
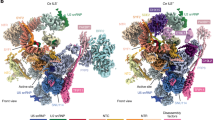
Extended Data Figure 5 Fitting of protein components into tri-snRNP map.
a, Prp8(885–2,413) crystal structure10 (PDB 4I43, green) and additional helices built de novo assigned to the N terminus of Prp8 (blue). b, Brr2–Jab1/MPN complex31 (PDB 4BGD). c, Snu114 homology model based on EF2 (ref. 26). d, The Prp6 TPR motifs built into the tri-snRNP map. e, U5 Sm proteins (grey) with Sm site (blue) based on the human U4 Sm structure (PDB 4WZJ). f, Dib1 (ref. 29) (PDB 1QGV). g, (i) Prp31. (ii), Comparison between the crystal structure of human Prp31(78–333) (ref. 33) (PDB 2OZB, grey) and that in tri-snRNP (yellow and blue). The coiled-coil domain (yellow) rotates by 60° in tri-snRNP with respect to the Nop domain (grey). Additional helices (blue) that extend from the N and C termini were built. h, U4 Sm proteins with part of U4 snRNA (blue) based on the human U4 Sm structure. i, Prp3 model. The ferredoxin-like domain was obtained from homology modelling while the extra helices were built de novo. j, Prp4 WD40 homology model with the extra helices built de novo. k, Snu13 (ref. 64) (PDB 2ALE). l, U6 LSm proteins67 (PDB 4M77).
Extended Data Figure 6 Fitting of the RNA components in tri-snRNP map.
a, c, The sequences and predicted secondary structures of U4/U6 snRNA and the long version of U5 snRNA, respectively. b, d, The maps of the fitted parts of U4/U6 snRNA and U5 snRNA, respectively. Unmodelled density assigned to U5 snRNA is also shown in d.
Extended Data Figure 7 Sequence alignment of yeast and human Snu114 with yeast and human elongation factor 2 (EF-2).
The secondary structures of our homology model for yeast Snu114 and the yeast EF-2 (ref. 26) (PDB 1N0V) are shown on the top and bottom of the alignment, respectively. Important sequence elements are also shown. The greyscale shading indicates the level of sequence conservation. A higher level of conservation is shown in a darker shade.
Extended Data Figure 8 The effect of ATP on Brr2-TAPS purified tri-snRNP.
a, Ethidium-bromide-stained native agarose gel (0.5%) showing the effects of ATP addition to Brr2-TAPS purified tri-snRNP used in this study. Upon ATP addition either without or with GTP/GDP, tri-snRNP fell apart (lanes 1–4). Under the same conditions, the addition of ADP or the non-hydrolysable ATP-analogue, AMPPNP, had no effects on the complex (lanes 5, 6). b, c, The effect of ATP addition observed by negative stain microscopy. When ATP was not present, tri-snRNP particles could be observed. When ATP was added to the sample before grid preparations, tri-snRNP particles fell apart as observed by many small components on the micrograph rather than tri-snRNP particles. d, Tri-snRNP model where U4/U6 snRNP proteins are not shown. In tri-snRNP, Brr2–Prp8Jab complex is loosely associated to the remaining U5 snRNP components including Prp8large, Prp8RNaseH, Prp8Nterm, Snu114, Dib1, U5 Sm proteins and U5 snRNA. After U4/U6 snRNA unwinding by Brr2, Brr2–Prp8Jab could be repositioned within the spliceosome. e, A schematic showing the arrangement of tri-snRNP protein and RNA components.
Supplementary information
The architecture of the spliceosomal U4/U6.U5 tri-snRNP
The video sequences showing the cryoEM density at two different contour levels; tri-snRNP map with all modeled components; fitting of available crystal structures into the cryoEM density: Brr2-Jab1/MPN (Prp8) complex31, Prp8 RNase H and large domains10, U4 and U5 Sm core domains65, Lsm core domain67 fitted into the multi-body map, Snu13 (ref. 64), human Prp31 (ref. 33) with remodeling, human Dib1 (ref. 29); fitting of homology models: Snu114 based translation factor EF2 (ref. 26), WD40 domain of Prp4, ferredoxin-like domain of Prp3 (ref. 36), TPR domain of Prp6; fitting of double helical RNA of the U4/U6 snRNA duplex and U5 snRNA; fitting of α-helices attributed to the N-terminal domain Prp8, Prp3 and Prp4; near complete pseudo-atomic structure of the yeast U4/U6.U5 tri-snRNP. (MOV 42926 kb)
Rights and permissions
About this article
Cite this article
Nguyen, T., Galej, W., Bai, Xc. et al. The architecture of the spliceosomal U4/U6.U5 tri-snRNP. Nature 523, 47–52 (2015). https://doi.org/10.1038/nature14548
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14548