Abstract
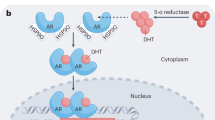
Prostate cancer resistance to castration occurs because tumours acquire the metabolic capability of converting precursor steroids to 5α-dihydrotestosterone (DHT), promoting signalling by the androgen receptor and the development of castration-resistant prostate cancer1,2,3. Essential for resistance, DHT synthesis from adrenal precursor steroids or possibly from de novo synthesis from cholesterol commonly requires enzymatic reactions by 3β-hydroxysteroid dehydrogenase (3βHSD), steroid-5α-reductase (SRD5A) and 17β-hydroxysteroid dehydrogenase (17βHSD) isoenzymes4,5. Abiraterone, a steroidal 17α-hydroxylase/17,20-lyase (CYP17A1) inhibitor, blocks this synthetic process and prolongs survival6,7. We hypothesized that abiraterone is converted by an enzyme to the more active Δ4-abiraterone (D4A), which blocks multiple steroidogenic enzymes and antagonizes the androgen receptor, providing an additional explanation for abiraterone’s clinical activity. Here we show that abiraterone is converted to D4A in mice and patients with prostate cancer. D4A inhibits CYP17A1, 3βHSD and SRD5A, which are required for DHT synthesis. Furthermore, competitive androgen receptor antagonism by D4A is comparable to the potent antagonist enzalutamide. D4A also has more potent anti-tumour activity against xenograft tumours than abiraterone. Our findings suggest an additional explanation—conversion to a more active agent—for abiraterone’s survival extension. We propose that direct treatment with D4A would be more clinically effective than abiraterone treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Titus, M. A., Schell, M. J., Lih, F. B., Tomer, K. B. & Mohler, J. L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 11, 4653–4657 (2005)
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008)
Chang, K. H. et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell 154, 1074–1084 (2013)
Knudsen, K. E. & Penning, T. M. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol. Metab. 21, 315–324 (2010)
Chang, K. H. & Sharifi, N. Prostate cancer-from steroid transformations to clinical translation. Nature Rev. Urol. 9, 721–724 (2012)
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011)
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013)
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012)
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014)
Li, R. et al. Abiraterone inhibits 3β-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer. Clin. Cancer Res. 18, 3571–3579 (2012)
Ferraldeschi, R., Sharifi, N., Auchus, R. J. & Attard, G. Molecular pathways: inhibiting steroid biosynthesis in prostate cancer. Clin. Cancer Res. 19, 3353–3359 (2013)
Garrido, M. et al. A-ring modified steroidal azoles retaining similar potent and slowly reversible CYP17A1 inhibition as abiraterone. J. Steroid Biochem. Mol. Biol. 143, 1–10 (2014)
Chang, K. H. et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl Acad. Sci. USA 108, 13728–13733 (2011)
Richards, J. et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 72, 2176–2182 (2012)
Hodgson, M. C. et al. The androgen receptor recruits nuclear receptor CoRepressor (N-CoR) in the presence of mifepristone via its N and C termini revealing a novel molecular mechanism for androgen receptor antagonists. J. Biol. Chem. 280, 6511–6519 (2005)
Attard, G. et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 (2008)
Carreira, S. et al. Tumor clone dynamics in lethal prostate cancer. Sci. Transl. Med. 6, 254ra125 (2014)
Balk, S. Emerging developments in abiraterone resistance. In Proc. American Association for Cancer Research Prostate Cancer Foundation Conference on Advances in Prostate Cancer Research. Abstr. IA11 (American Association for Cancer Research, 2014)
Attard, G. et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J. Clin. Endocrinol. Metab. 97, 507–516 (2012)
Efstathiou, E. et al. Enzalutamide (ENZA) in combination with abiraterone acetate (AA) in bone metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. 32, abstr. 5000. (2014)
Fizazi, K. et al. Phase III, randomized, double-blind, multicenter trial comparing orteronel (TAK-700) plus prednisone with placebo plus prednisone in patients with metastatic castration-resistant prostate cancer that has progressed during or following docetaxel-based therapy: ELM-PC 5. J. Clin. Oncol. 33, 723–731 (2015)
Papari-Zareei, M., Brandmaier, A. & Auchus, R. J. Arginine 276 controls the directional preference of AKR1C9 (rat liver 3α-hydroxysteroid dehydrogenase) in human embryonic kidney 293 cells. Endocrinology 147, 1591–1597 (2006)
Li, Z., Nie, F., Wang, S. & Li, L. Histone H4 Lys 20 monomethylation by histone methylase SET8 mediates Wnt target gene activation. Proc. Natl Acad. Sci. USA 108, 3116–3123 (2011)
Evaul, K., Li, R., Papari-Zareei, M., Auchus, R. J. & Sharifi, N. 3β-hydroxysteroid dehydrogenase is a possible pharmacological target in the treatment of castration-resistant prostate cancer. Endocrinology 151, 3514–3520 (2010)
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009)
Kushnir, M. M. et al. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin. Chem. 56, 1138–1147 (2010)
Acknowledgements
This work has been supported in part by funding from a Howard Hughes Physician-Scientist Early Career Award (to N.S.), by the Prostate Cancer Foundation (to N.S.), by an American Cancer Society Research Scholar Award (12-038-01-CCE; to N.S.), grants from the US Army Medical Research and Materiel Command (PC080193 to N.S. and PC121382 to Z.L.), and additional grants from the National Cancer Institute (R01CA168899, R01CA172382, and R01CA190289; to N.S.).
Author information
Authors and Affiliations
Contributions
Z.L. performed gene expression, metabolism, chromatin immunoprecipitation (ChIP) and mouse xenograft studies. A.B., M.A. and D.B. performed mass spectrometry studies. J.A.G. and R.D. participated in clinical studies. J.L. and S.K.U. performed enzymology studies, and S.K.U. also performed chemical syntheses. Z.L., R.J.A. and N.S. designed the studies and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 D4A is detectable in patients with prostate cancer treated with Abi acetate.
a, Mass spectrometry tracings of Abi and D4A in the serum of 12 patients treated with Abi acetate. Blood was drawn between 2 and 14 h after the administration of the 1,000 mg daily dose. b, Duration of Abi acetate therapy and time from last dose to blood draw for individual patients.
Extended Data Figure 2 3βHSD converts Abi to D4A.
a, 3βHSD1 expression permits catalysis of DHEA to AD. LAPC4 cells were transfected with 3βHSD1 or vector and treated with [3H]DHEA. Medium was collected 24 h later and androgens were separated and quantified by HPLC. b, 3βHSD1 expression allows conversion of Abi to D4A. LAPC4 cells were transfected with 3βHSD1 or vector and then treated with Abi. Medium was collected after 24 h and D4A and Abi were separated by HPLC. c, 3βHSD enzymatic activity present in the mouse adrenal gland but not prostate gland converts Abi to D4A. Mouse adrenal and prostate glands were harvested and minced before culturing in the presence of media containing Abi. Medium was collected after 24 h for separation and quantitation of D4A and Abi by HPLC.
Extended Data Figure 3 D4A inhibits 3βHSD1 activity.
a, D4A inhibits 3βHSD1 activity in LNCaP. Cells were treated with [3H]DHEA (DHEA: 100nM; [3H]DHEA, 1,000,000 c.p.m. per well) with 0.1, 1 or 10 µM D4A and Abi, for 9 and 24 h. DHEA and AD were separated and quantified by TLC. ImageJ was used to quantify steroids. For ease of comparison, arrows denote AD percentage for 0.1 µM D4A and 1 µM Abi treatment groups. b, VCaP cells were treated with [3H]DHEA and the indicated concentrations of D4A and Abi. The percentages of DHEA and AD were determined by HPLC. Experiments were performed with biological replicates (n = 3) and results are shown as mean ± s.d.
Extended Data Figure 4 D4A has a higher affinity for both mutant-type AR (LNCaP cells) and wild-type AR (LAPC4 cells) than abiraterone (Abi) and bicalutamide (Bic) and inhibits AR chromatin occupancy better than Abi.
a, Competition plots for D4A, Abi and Bic. b, Competition plots for unlabelled R1881 and D4A. Displacement of [3H]R1881 is described in Methods. Experiments were performed with biological replicates (n = 3) and results are shown as mean ± s.d. c, D4A inhibition of AR chromatin occupancy is superior to Abi. LNCaP cells were treated with the indicated concentrations of DHT, D4A, Abi and enzalutamide (Enz) for 3 h. AR chromatin occupancy for PSA, TMPRSS2 and FKBP5 was detected with ChIP. Experiments were performed with technical replicates (n = 3) and results are shown as mean ± s.d. All experiments were repeated independently at least three times.
Extended Data Figure 5 D4A inhibits expression of androgen-responsive genes.
a, D4A inhibits PSA expression in LAPC4 cells. Cells were treated with DHT (0.5 nM), DHEA (40 nM) or R1881 (0.1 nM) with or without Abi or D4A (1 µM) for 24 h. b, D4A inhibits PSA expression in LAPC4 in a dose-dependent manner. c, D4A inhibits AR target gene expression in C4-2 cells. Cells were treated with vehicle control (Ctrl), DHT (0.5 nM) or DHEA (40 nM) with or without Abi (1 µM), D4A (1 µM) or Enz (1 µM) for 24 h. d, D4A is comparable to Enz in inhibiting DHT-induced target gene expression in VCaP cells. Gene expression was assessed in triplicate, detected by qPCR and normalized to RPLP0. Experiments were performed with technical replicates (n = 3) and results are shown as mean ± s.d. All experiments were repeated independently at least three times.
Extended Data Figure 6 D4A impedes VCaP xenograft growth.
*P < 0.05 and **P < 0.01 for the difference between D4A (n = 10 mice) and AA (n = 10 mice) treatment groups. N = 9 mice for the control group.
Extended Data Figure 7 D4A does not increase deoxycorticosterone concentrations.
Serum of mice undergoing long-term treatment with D4A was collected and deoxycorticosterone concentrations were determined by liquid chromatography–mass spectrometry. Compared with control mice injected with vehicle, D4A does not increase deoxycorticosterone concentrations. For both D4A and control groups, n = 9 biological replicates (mice).
Rights and permissions
About this article
Cite this article
Li, Z., Bishop, A., Alyamani, M. et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature 523, 347–351 (2015). https://doi.org/10.1038/nature14406
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14406