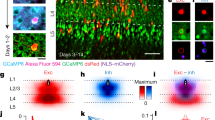
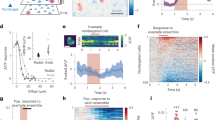
Abstract
The strength of synaptic connections fundamentally determines how neurons influence each other’s firing. Excitatory connection amplitudes between pairs of cortical neurons vary over two orders of magnitude, comprising only very few strong connections among many weaker ones1,2,3,4,5,6,7,8,9. Although this highly skewed distribution of connection strengths is observed in diverse cortical areas1,2,3,4,5,6,7,8,9, its functional significance remains unknown: it is not clear how connection strength relates to neuronal response properties, nor how strong and weak inputs contribute to information processing in local microcircuits. Here we reveal that the strength of connections between layer 2/3 (L2/3) pyramidal neurons in mouse primary visual cortex (V1) obeys a simple rule—the few strong connections occur between neurons with most correlated responses, while only weak connections link neurons with uncorrelated responses. Moreover, we show that strong and reciprocal connections occur between cells with similar spatial receptive field structure. Although weak connections far outnumber strong connections, each neuron receives the majority of its local excitation from a small number of strong inputs provided by the few neurons with similar responses to visual features. By dominating recurrent excitation, these infrequent yet powerful inputs disproportionately contribute to feature preference and selectivity. Therefore, our results show that the apparently complex organization of excitatory connection strength reflects the similarity of neuronal responses, and suggest that rare, strong connections mediate stimulus-specific response amplification in cortical microcircuits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Markram, H., Lübke, J., Frotscher, M., Roth, A. & Sakmann, B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. (Lond.) 500, 409–440 (1997)
Feldmeyer, D., Lübke, J. & Sakmann, B. Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J. Physiol. (Lond.) 575, 583–602 (2006)
Holmgren, C., Harkany, T., Svennenfors, B. & Zilberter, Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J. Physiol. (Lond.) 551, 139–153 (2003)
Song, S., Sjöström, P. J., Reigl, M., Nelson, S. & Chklovskii, D. B. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, e68 (2005)
Lefort, S., Tomm, C., Floyd Sarria, J. & Petersen, C. H. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron 61, 301–316 (2009)
Buzsáki, G. & Mizuseki, K. The log-dynamic brain: how skewed distributions affect network operations. Nature Rev. Neurosci. 15, 264–278 (2014)
Morishima, M., Morita, K., Kubota, Y. & Kawaguchi, Y. Highly differentiated projection-specific cortical subnetworks. J. Neurosci. 31, 10380–10391 (2011)
Levy, R. B. & Reyes, A. D. Spatial profile of excitatory and inhibitory synaptic connectivity in mouse primary auditory cortex. J. Neurosci. 32, 5609–5619 (2012)
Tarczy-Hornoch, K., Martin, K. A. C., Stratford, K. J. & Jack, J. J. Intracortical excitation of spiny neurons in layer 4 of cat striate cortex in vitro. Cereb. Cortex 9, 833–843 (1999)
Ko, H. et al. Functional specificity of local synaptic connections in neocortical networks. Nature 473, 87–91 (2011)
Stosiek, C., Garaschuk, O., Holthoff, K. & Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl Acad. Sci. USA 100, 7319–7324 (2003)
Smith, S. L. & Häusser, M. Parallel processing of visual space by neighboring neurons in mouse visual cortex. Nature Neurosci. 13, 1144–1149 (2010)
Bonin, V., Histed, M. H., Yurgenson, S. & Reid, R. C. Local diversity and fine-scale organization of receptive fields in mouse visual cortex. J. Neurosci. 31, 18506–18521 (2011)
Niell, C. M. & Stryker, M. P. Highly selective receptive fields in mouse visual cortex. J. Neurosci. 28, 7520–7536 (2008)
Ko, H. et al. The emergence of functional microcircuits in visual cortex. Nature 496, 96–100 (2013)
Carandini, M. et al. Do we know what the early visual system does? J. Neurosci. 25, 10577–10597 (2005)
Perin, R., Berger, T. K. & Markram, H. A synaptic organizing principle for cortical neuronal groups. Proc. Natl Acad. Sci. USA 108, 5419–5424 (2011)
Reid, R. C. & Alonso, J. M. Specificity of monosynaptic connections from thalamus to visual cortex. Nature 378, 281–284 (1995)
Lien, A. D. & Scanziani, M. Tuned thalamic excitation is amplified by visual cortical circuits. Nature Neurosci. 16, 1315–1323 (2013)
Li, Y.-t., Ibrahim, L. A., Liu, B.-h., Zhang, L. I. & Tao, H. W. Linear transformation of thalamocortical input by intracortical excitation. Nature Neurosci. 16, 1324–1330 (2013)
Tan, A. Y. Y., Brown, B. D., Scholl, B., Mohanty, D. & Priebe, N. J. Orientation selectivity of synaptic input to neurons in mouse and cat primary visual cortex. J. Neurosci. 31, 12339–12350 (2011)
Jia, H., Rochefort, N. L., Chen, X. & Konnerth, A. Dendritic organization of sensory input to cortical neurons in vivo. Nature 464, 1307–1312 (2010)
Smith, S. L., Smith, I., Branco, T. & Häusser, M. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature 503, 115–120 (2013)
Clopath, C., Büsing, L., Vasilaki, E. & Gerstner, W. Connectivity reflects coding: a model of voltage-based STDP with homeostasis. Nature Neurosci. 13, 344–352 (2010)
Druckmann, S. & Chklovskii, D. B. Neuronal circuits underlying persistent representations despite time varying activity. Curr. Biol. 22, 2095–2103 (2012)
Li, L.-y., Li, Y.-t., Zhou, M., Tao, H. W. & Zhang, L. I. Intracortical multiplication of thalamocortical signals in mouse auditory cortex. Nature Neurosci. 16, 1179–1181 (2013)
Douglas, R. J., Koch, C., Mahowald, M., Martin, K. A. & Suarez, H. H. Recurrent excitation in neocortical circuits. Science 269, 981–985 (1995)
Ben-Yishai, R., Bar-Or, R. L. & Sompolinsky, H. Theory of orientation tuning in visual cortex. Proc. Natl Acad. Sci. USA 92, 3844–3848 (1995)
Somers, D. C., Nelson, S. B. & Sur, M. An emergent model of orientation selectivity in cat visual cortical simple cells. J. Neurosci. 15, 5448–5465 (1995)
Mrsic-Flogel, T. D. et al. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron 54, 961–972 (2007)
Nimmerjahn, A., Kirchhoff, F., Kerr, J. N. D. & Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nature Methods 1, 1–7 (2004)
Brainard, D. H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997)
Pelli, D. G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442 (1997)
Vogelstein, J. T. et al. Fast nonnegative deconvolution for spike train inference from population calcium imaging. J. Neurophysiol. 104, 3691–3704 (2010)
Hofer, S. B. et al. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nature Neurosci. 14, 1045–1052 (2011)
Margrie, T. W., Brecht, M. & Sakmann, B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 444, 491–498 (2002)
Smyth, D., Willmore, B., Baker, G. E., Thompson, I. D. & Tolhurst, D. J. The receptive-field organization of simple cells in primary visual cortex of ferrets under natural scene stimulation. J. Neurosci. 23, 4746–4759 (2003)
Acknowledgements
We thank K. Harris, G. Keller, T. Margrie, J. Sjöström, P. Znamenskiy and our laboratory members for discussions and comments, and T. Margrie, E. Rancz and J. Poulet for advice on in vivo whole-cell recordings. This work was supported by the Wellcome Trust (grant no. 095074) and the European Research council. L.C. was funded by the 4-year PhD programme in Neuroscience at UCL. M.F.I. was funded by a UCL International PhD fellowship. D.R.M. was supported by the University of Basel Young Researchers fund.
Author information
Authors and Affiliations
Contributions
L.C., M.F.I., S.B.H. and T.D.M.-F. designed the experiments. L.C. and M.F.I. performed the in vivo and in vitro experiments. R.H. and E.N.S. performed the in vivo whole-cell recordings. H.K. and S.B.H. contributed to the transition during in vivo and in vitro experiments. H. K. and L.C. wrote the software for whole-cell recordings in vivo and in vitro. L.C., M.F.I. and D.R.M. analysed the data. L.C., M.F.I., D.R.M. and T.D.M.-F. wrote the manuscript. All authors discussed the data and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Relationship between response correlation coefficient or RF correlation and cortical distance.
a, Pairwise response correlation coefficient plotted as a function of cortical distance, for an example region, indicates only a weak relationship between response correlation and cortical distance (R = −0.06). Red line denotes mean value of response correlation in 50 μm bins of cortical distance. b, Pairwise RF correlation plotted as a function of cortical distance, for the same example region as in a. Again, only a weak relationship was observed (R = −0.02).
Extended Data Figure 2 Relationship between mean connection amplitude and response correlation or RF correlation.
a, Black trace, mean connection amplitude (excluding unconnected pairs) plotted against response correlation. Dashed grey line indicates mean EPSP amplitude of all connections. Grey shaded region represents the 95% confidence interval of the expected mean, estimated by repeated random reshuffling of the EPSP amplitudes among all cell pairs in the data set. Connections were binned with ranges from −0.1 to 0, 0 to 0.1, and so on. b, Black trace, mean connection amplitude (excluding unconnected pairs) plotted against RF correlation. Dashed grey line indicates mean EPSP amplitude of all connections. Grey shaded region represents the 95% confidence interval of the expected mean, estimated by repeated random reshuffling of the EPSP amplitudes among all cell pairs in the data set. Connections were binned with ranges from −0.8 to −0.6, −0.6 to −0.4, and so on.
Extended Data Figure 3 Relationship between connectivity and RF subfield overlap.
a, The amount of ON and OFF subfield overlap (see Methods) was strongly correlated to the overall RF similarity as measured by RF correlation (R = 0.79, P < 1 × 10−10). b, Left panel, connection probability increased with increasing ON subfield overlap (P = 0.05; Cochran–Armitage test). Middle panel, EPSP amplitudes categorized into bins of ON overlap. Black line, median EPSP amplitude for each bin. Right panel, EPSP amplitude plotted against ON overlap. Red data points, bidirectional connections. Black data points, unidirectional connections. Underlying histogram shows frequency of recorded cell pairs as a function of ON overlap. c, Same as b, but for OFF overlap (P = 0.002; Cochran–Armitage test). d, Same as b, but for combined ON and OFF overlap (P = 1.8 × 10−5; Cochran–Armitage test). P values from the Cochran–Armitage test. To perform the Cochran–Armitage test, the bins at 0 and >0–0.15 were considered together, so that groups were evenly spaced.
Extended Data Figure 4 Similarity of shared neuronal properties ranked according to how well they predict connection amplitude, when excluding unconnected pairs.
Prediction performance and P values were calculated using a Monte-Carlo analysis (see Methods). Colours of the discs indicate P values.
Extended Data Figure 5 Relationship between bidirectional and unidirectional connections and RF properties.
a, EPSP amplitude plotted against RF correlation from bidirectionally (red) and unidirectionally connected pairs (black). Replotted from Fig. 2g. b, EPSP amplitude for bi- or unidirectional connections. Bidirectional connections were stronger than unidirectional connections (median connection amplitude: 0.44 mV for bidirectional connections, n = 22; 0.16 mV for unidirectional connections, n = 50; P = 4.4 × 10−4, Wilcoxon rank-sum test). c, RF correlation for bidirectionally connected, unidirectionally connected and unconnected pairs. The RFs of bidirectionally connected pairs were more correlated than those of unidirectionally connected or unconnected pairs (median RF correlation: 0.3 for bidirectionally connected pairs, n = 11; 0.04 for unidirectionally connected pairs, n = 50; P = 0.002; and −0.02 for unconnected pairs, n = 191, P = 5.3 × 10−5), although unidirectionally connected pairs did not have higher RF correlations than unconnected pairs (P = 0.18, Wilcoxon rank-sum test). d, Mean EPSP amplitude versus RF correlation for all (yellow), unidirectionally (black) or bidirectionally (red) connected pairs. There was a positive relationship between RF correlation and connection amplitude for both unidirectional and bidirectional connections.
Extended Data Figure 6 Method of RF normalization.
a, We normalized postsynaptic RFs to a template RF that was a vertical Gabor with 0 degree phase and an arbitrary but fixed spatial frequency (far right). A Gabor was fit to the RF of each postsynaptic neuron, and then rotated, translated and scaled so that the ON subfield was centred on the template’s ON subfield and the spatial frequencies matched. The same transformation was applied to presynaptic RFs of any simultaneously patched neurons. b, Transformation of the RF from an example postsynaptic neuron (upper row), and for the RF for its connected presynaptic neuron (middle row). Bottom row shows presynaptic RF outline overlaid on the postsynaptic RF at each step in the transformation.
Extended Data Figure 7 Overlay of RFs between connected neurons.
Presynaptic RF outline overlaid on the postsynaptic RF for all the connected pairs after performing normalization of the pre- and postsynaptic RFs to the RF template (n = 45). Numbers indicate connection amplitude.
Extended Data Figure 8 Overlay of RFs between unconnected neurons.
Assessed presynaptic RF outlines overlaid on the assessed postsynaptic RF for a representative set of unconnected pairs after normalization to the RF template.
Extended Data Figure 9 Contribution of strong and weak connections to membrane potential depolarization.
Removal of an increasingly larger fraction of the strongest inputs from the L2/3 model steeply reduces the large modulation component (F1) but more gradually reduces the mean depolarization component (F0). Model from Fig. 4d. Purple arrow indicates the weakest 75% of connections, as shown in Fig. 4i, j.
Rights and permissions
About this article
Cite this article
Cossell, L., Iacaruso, M., Muir, D. et al. Functional organization of excitatory synaptic strength in primary visual cortex. Nature 518, 399–403 (2015). https://doi.org/10.1038/nature14182
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14182