Abstract
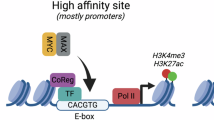
In mammalian cells, the MYC oncoprotein binds to thousands of promoters1,2,3,4. During mitogenic stimulation of primary lymphocytes, MYC promotes an increase in the expression of virtually all genes1. In contrast, MYC-driven tumour cells differ from normal cells in the expression of specific sets of up- and downregulated genes that have considerable prognostic value5,6,7. To understand this discrepancy, we studied the consequences of inducible expression and depletion of MYC in human cells and murine tumour models. Changes in MYC levels activate and repress specific sets of direct target genes that are characteristic of MYC-transformed tumour cells. Three factors account for this specificity. First, the magnitude of response parallels the change in occupancy by MYC at each promoter. Functionally distinct classes of target genes differ in the E-box sequence bound by MYC, suggesting that different cellular responses to physiological and oncogenic MYC levels are controlled by promoter affinity. Second, MYC both positively and negatively affects transcription initiation independent of its effect on transcriptional elongation8. Third, complex formation with MIZ1 (also known as ZBTB17)9 mediates repression of multiple target genes by MYC and the ratio of MYC and MIZ1 bound to each promoter correlates with the direction of response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
ArrayExpress
Gene Expression Omnibus
Data deposits
Microarray data sets have been deposited in ArrayExpress under accession number E-MTAB-1886. All ChIP- and RNA-sequencing data sets have been deposited in the Gene Expression Omnibus under accession number GSE44672.
References
Nie, Z. et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 151, 68–79 (2012)
Lin, C. Y. et al. Transcriptional amplification in tumor cells with Elevated c-Myc. Cell 151, 56–67 (2012)
Chen, X. et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117 (2008)
Zeller, K. I. et al. Global mapping of c-Myc binding sites and target gene networks in human B cells. Proc. Natl Acad. Sci. USA 103, 17834–17839 (2006)
Horiuchi, D. et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J. Exp. Med. 209, 679–696 (2012)
Yustein, J. T. et al. Induction of ectopic Myc target gene JAG2 augments hypoxic growth and tumorigenesis in a human B-cell model. Proc. Natl Acad. Sci. USA 107, 3534–3539 (2010)
Valentijn, L. J. et al. Functional MYCN signature predicts outcome of neuroblastoma irrespective of MYCN amplification. Proc. Natl Acad. Sci. USA 109, 19190–19195 (2012)
Rahl, P. B. et al. c-Myc regulates transcriptional pause release. Cell 141, 432–445 (2010)
Wiese, K. E. et al. The role of MIZ-1 in MYC-dependent tumorigenesis. Cold Spring Harb. Perspect. Med. 3, a014290 (2013)
Evan, G. I. et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69, 119–128 (1992)
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005)
Blackwell, T. K. et al. Binding of Myc proteins to canonical and noncanonical DNA sequences. Mol. Cell. Biol. 13, 5216–5224 (1993)
Eberhardy, S. R. & Farnham, P. J. c-Myc mediates activation of the cad promoter via a post-RNA polymerase II recruitment mechanism. J. Biol. Chem. 276, 48562–48571 (2001)
Eberhardy, S. R. & Farnham, P. J. Myc recruits P-TEFb to mediate the final step in the transcriptional activation of the cad promoter. J. Biol. Chem. 277, 40156–40162 (2002)
Guccione, E. et al. Myc-binding-site recognition in the human genome is determined by chromatin context. Nature Cell Biol. 8, 764–770 (2006)
Bouchard, C., Marquardt, J., Bras, A., Medema, R. H. & Eilers, M. Myc-induced proliferation and transformation require Akt-mediated phosphorylation of FoxO proteins. EMBO J. 23, 2830–2840 (2004)
Hsin, J. P. & Manley, J. L. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 26, 2119–2137 (2012)
Wolf, E. et al. Miz1 is required to maintain autophagic flux. Nature Commun. 4, 2535 (2013)
Staller, P. et al. Repression of p15INK4b expression by Myc through association with Miz-1. Nature Cell Biol. 3, 392–399 (2001)
Iraci, N. et al. A SP1/MIZ1/MYCN repression complex recruits HDAC1 at the TRKA and p75NTR promoters and affects neuroblastoma malignancy by inhibiting the cell response to NGF. Cancer Res. 71, 404–412 (2011)
Gartel, A. L. et al. Myc represses the p21(WAF1/CIP1) promoter and interacts with Sp1/Sp3. Proc. Natl Acad. Sci. USA 98, 4510–4515 (2001)
Herold, S. et al. Negative regulation of the mammalian UV response by Myc through association with Miz-1. Mol. Cell 10, 509–521 (2002)
Kosan, C. et al. Transcription factor Miz-1 is required to regulate interleukin-7 receptor signaling at early commitment stages of B cell differentiation. Immunity 33, 917–928 (2010)
van Riggelen, J. et al. The interaction between Myc and Miz1 is required to antagonize TGFβ-dependent autocrine signaling during lymphoma formation and maintenance. Genes Dev. 24, 1281–1294 (2010)
Hingorani, S. R. et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 (2005)
de Alboran, I. M. et al. Analysis of c-Myc function in normal cells via conditional gene-targeted mutation. Immunity 14, 45–55 (2001)
Naldini, L., Blomer, U., Gage, F. H., Trono, D. & Verma, I. M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl Acad. Sci. USA 93, 11382–11388 (1996)
Boyd, K. E. & Farnham, P. J. Myc versus USF: discrimination at the cad gene is determined by core promoter elements. Mol. Cell. Biol. 17, 2529–2537 (1997)
Langmead, B. Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinformatics 32, 11.7.1–11.7.14 (2010)
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008)
Nicol, J. W., Helt, G. A., Blanchard, S. G., Jr, Raja, A. & Loraine, A. E. The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics 25, 2730–2731 (2009)
Ye, T. et al. seqMINER: an integrated ChIP-seq data interpretation platform. Nucleic Acids Res. 39, e35 (2011)
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010)
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010)
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols 4, 44–57 (2009)
Machanick, P. & Bailey, T. L. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27, 1696–1697 (2011)
Barski, A. et al. Pol II and its associated epigenetic marks are present at Pol III-transcribed noncoding RNA genes. Nature Struct. Mol. Biol. 17, 629–634 (2010)
Grandori, C. et al. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nature Cell Biol. 7, 311–318 (2005)
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG) through grants 222/5-3 and 222/12-1 (to M.E.), by a stipend of the graduate college 1048 (“Molecular basis of organ development in vertebrates” to S.W.) and through the DFG Research Center for Experimental Biomedicine (to E.W.). M.T. was supported by grants from the Institut National Du Cancer (INCa) and by the Ligue National Contre le Cancer (Equipe Labellisée). O.S. and J.M. are funded by a Cancer Research UK core grant and a European Research Council investigator grant, “Coloncan”. We thank Y. L. Lee and T. Poh for help with ChIP-sequencing, F. Finkernagel for help with the bioinformatic analysis, A. Au for help with mouse experiments, B. Lüscher for critical reading of the manuscript and D. Levens for providing data before publication.
Author information
Authors and Affiliations
Contributions
S.W., F.L., E.W., K.E.W., B.v.E. and T.W. performed the experiments, S.H., H.D.-O. and M.T. characterized the MIZ1 637–807 antibody, S.W., F.L. and E.W. performed ChIP-sequencing experiments, J.M. and S.K. analysed the pancreas model, F.L., F.R., M.B., M.F., S.W. and L.R. performed statistical analyses. E.W., L.Z., O.S., C.-L.W. and M.E. devised and supervised experiments. E.W. and M.E. wrote the paper and should be considered as senior authors of this study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Characterization of U2OS cells expressing a doxycycline-inducible allele of MYC.
a, RT–qPCR analyses demonstrating induction of MYC mRNA in response to doxycycline (Dox; 30 h; 1 μg ml−1). Error bars show s.d. of triplicate technical assays. Control cells were treated with ethanol. b, Immunofluorescence using anti-MYC antibody and Hoechst staining of U2OS cells before and 30 h after induction of MYC. Original magnification: ×200. c, Cell size of U2OS cells before and 30 h after induction of MYC. Size was calculated from measurements of forward scatter (FSC) in arbitrary units. Errors bars show s.d. (n = 3; unless indicated otherwise, n indicates the number of independent experiments in Extended Data Figs 2–10 legends). d, Percentage of BrdU-positive U2OS cells (left) and FACS analysis (right) documenting cell cycle distribution before and 30 h after induction of MYC with doxycycline (n = 3). e, Phase contrast pictures documenting induction of apoptosis by MYC. Pictures were taken 72 h after addition of doxycycline. Original magnification: ×50. f, Amount of total RNA (left) and of mRNA (right) per cell before and after induction of MYC. Error bars show standard error of the mean (s.e.m.) (n = 4).
Extended Data Figure 2 Controls for antibody specificity and validation of ChIP-sequencing data.
a, HeLa cells were transfected with either control vectors or expression vectors encoding human MIZ1. Cell lysates were probed with the anti-MIZ1 antibodies used in this study. b, Immunoblot documenting specificity of the MIZ1 (637–803) antibody used for ChIP-sequencing experiments. The blot is from nuclear extracts of IMR90 human fibroblasts. c, Immunoprecipitations documenting that endogenous MIZ1 immunoprecipitated from HeLa cells by the MIZ1 (637–803) antibody is recognized by the N17 and 10E2 anti-MIZ1 antibodies. d, MIZ1 ChIP data for 71 randomly selected MIZ1-binding sites and 4 negative controls. Shown is the percentage of input DNA precipitated by the MIZ1 (637–803) antibody used for ChIP-sequencing and control IgG. Validation with a second MIZ1 antibody (10E2) is shown below. Minus sign indicates a non-significant enrichment over IgG (q < 0.05, heteroscedastic t-test). The arrow points to the start site of the CDKN1A gene (‘p21-5’), a known target site of MIZ1. e, ChIP experiments analysing binding of MYC to randomly chosen promoters identified in the ChIP-sequencing analysis and four negative control sites. The experiment was performed using the antibody N262 in HeLa cells.
Extended Data Figure 3 Binding of MYC to chromatin and MYC-dependent changes in gene expression in U2OS cells.
a, Distribution of MYC tags around the TSS of all human Pol II genes with and without induction of exogenous MYC. b, Example of MYC binding to a promoter and an intragenic enhancer. Enhancers are identified by the presence of H3K4me1 and H3K27ac and the absence of H3K4me3. Exons are indicated as vertical bars, the untranslated region (UTR) shown as a thick black line. c, Heat map documenting binding of MYC to all enhancers identified in U2OS cells. Enhancer positions are centred according to MYC occupancy within a window of ±1 kb of the centre of the enhancer region and are sorted according to the number of H3K4me1 tags. d, The diagram shows the MYC-induced change in expression (plotted as log2FC) versus total expression levels for all genes found in the RNA-sequencing as determined by RNA-sequencing. Red colour indicates significantly regulated genes (q < 0.01) (n = 3, biological replicates). CPM, counts per million. e, MYC-induced changes in gene expression are stereotypic. The panel shows a GSEA analysis of regulated genes in comparison to previously identified sets of MYC-regulated genes. f, Enrichment plot of a GSEA analysis showing that MYC-activated genes from U2OS cells identify lung tumours that have amplified MYC.
Extended Data Figure 4 Oncogenic levels of MYC establish a tumour-cell-specific pattern of gene expression in HeLa cells.
a, Immunoblots documenting MYC levels upon infection of HeLa cells with either control or lentiviruses expressing shRNA-targeting MYC. All analyses are shown for pools of stably infected and selected cells. b, Cell size of control and MYC-depleted HeLa cells. Size was calculated from measurements of forward scatter (FSC) in arbitrary units. Errors bars show s.d. (n = 3). c, Cellular RNA content of control and MYC-depleted HeLa cells (n = 3). d, The panels show GSEA analyses of gene sets regulated upon depletion of MYC in HeLa cells demonstrating a close overlap with previously identified sets of MYC- and MYCN-regulated genes. e, Stratification of human lung tumours by genes regulated in response to MYC depletion in HeLa cells. Shown is a GSEA enrichment plot with a gene set defining MYC-amplified lung tumours. f, Antibodies used in this study.
Extended Data Figure 5 MYC recruitment at enhancer regions and gene regulation.
a, b, Diagram depicting the change in gene expression versus the change in occupancy (plotted as fold recruitment) upon induction of MYC expression for 3,766 enhancers that are bound by MYC and the nearest genes in U2OS cells. Activated (n = 1,457) (a) and repressed genes (n = 2,309) (b) are shown in each diagram with 25 equally sized bins. c, GO terms of 500 MYC-bound genes that show the smallest change in gene expression and of the 500 most strongly MYC-regulated genes. d, Schematic drawing summarizing the results. The annotations ‘cell growth’ and ‘transformation’ are meant to summarize the GO terms. e, MYC-dependent changes in histone H4 acetylation. The diagram summarizes the change in histone H4 acetylation at the promoters of all MYC-bound genes in response to induction of MYC in U2OS cells. Data are stratified by the MYC-induced change in expression (plotted as log2FC). Each dot represents median values of a bin of 386 genes.
Extended Data Figure 6 Additional characterization of MIZ1-binding sites on chromatin in HeLa cells.
a, Examples of ChIP-sequencing traces for two known MYC/MIZ1 target genes (CDKN2B, CEBPA) and INO80 (an E-box-containing promoter). b, Histogram showing the distribution of MIZ1-binding sites relative to TSSs. The positions of MIZ1 peaks are plotted relative to the next TSS of genes listed in the UCSC GoldenPath RefSeq database. c, Genome browser picture documenting binding of MYC and MIZ1 to a cluster of transfer (t)RNA genes (chr1:159,675,000–159,710,000 bp). ChIP-sequencing profiles for RNA Pol III binding are taken from ref. 37. Vertical lines indicate individual tRNA genes. d, Heat map documenting genome-wide occupancy of MYC, MIZ1 and RNA Pol III at tRNA loci. e, ChIP experiments documenting binding of MYC and MIZ1 to the indicated human tRNA genes. The graphs show a representative result with error bars depicting s.d. of technical triplicates from one representative experiment (n = 3). f, ChIP experiments analysing binding of MYC and MIZ1 to ribosomal (r)DNA clusters indicated by the oligonucleotide ‘H42.9’. The negative result for MIZ1 was confirmed using multiple primers to scan rDNA gene clusters, which previously has been shown to bind MYC38. Immunoprecipitations were performed in biological duplicates. The graphs show a representative result with error bars depicting s.d. of technical triplicates from one experiment. g, The heat map shows occupancy of 389 genomic regions predicted to be enhancers in HeLa cells. Enhancer regions were sorted according to p300 (GEO accession number GSM935500) occupancy and centred to the strongest MYC occupancy within a window of ±1 kb of the centre of the enhancer region.
Extended Data Figure 7 Characterization of two distinct classes of MYC/MIZ1-binding sites.
a, The diagram shows the number of tags for MYC and MIZ1 for the 1,000 most strongly MIZ1-bound core promoters transcribed by Pol II in HeLa cells. Red dots indicate binding sites that have a MIZ1 DNA-binding motif in the MIZ1 peak; green dots indicate binding sites that have an E-box (CACG/ATG) in the centre of the MYC peak (±60 bp). b, Gaussian Kernel distribution indicating the close relationship between MYC and MIZ1 peaks, with a bandwidth of 10 bp. Calculated was the distance between the summit of the MYC and the overlapping MIZ1 peak. The mean peak width (MYC, 687 bp; MIZ1,736 bp) is indicated by bars in the diagram. c, Re-ChIP experiment of MAX and MIZ1 at the VAMP4 promoter. Left, primary ChIP; right, re-ChIP. IgG, control IgG. Error bars show s.d. of technical triplicates (n = 2). d, ChIP experiments documenting binding of MIZ1 and MYC in control and MYC-depleted cells to the MACROD1 (E-box) and RSRC1 (MIZ1-binding motif) promoters. FBXW8 was used as a control for MYC depletion from chromatin on a gene that was not MIZ1-bound. To control for experimental variation, values are normalized relative to the amount of Pol II precipitated in parallel reactions and background IgG signals were subtracted. e, Recruitment of MIZ1 by MYC to joint binding sites in U2OS cells. Representative browser pictures are from U2OS cells before and after induction of MYC. f, ChIP experiment documenting MAX and MIZ1 binding to the RSRC1 (MIZ1-binding motif) promoter. The negative control region is on chromosome 11:79,556,864–79,556,942 bp.
Extended Data Figure 8 MIZ1 binding and transcriptional regulation by MYC.
a, RT–qPCR assays showing no change in expression of genes that have a MIZ1 consensus binding sequence in their promoter in response to addition of doxycycline to U2OS cells expressing a doxycycline-inducible allele of MYC. CAMKV and ALDH3B1 are MYC-regulated genes used as controls. b, Long-lived protein degradation assay in U2OS cells in the presence and absence of doxycycline. Where indicated, 3-methyladenin (3-MA) was added to inhibit autophagy. Values are normalized to 3-MA sample. Error bars show s.d. of biological replicates (n = 3). c, DREME analysis showing the most strongly enriched motifs in the 200 most MYC-activated and MYC-repressed genes. For the analysis, MYC peaks (±200 bp around the summit) that are located in promoters of MYC-regulated genes were used. d, Diagram depicting transcriptional activation of 2,787 genes upon induction of MYC in U2OS cells expressing control shRNA or shRNA targeting MIZ1 (r = 0.99; P = 3.9 × 10−25). Each bin summarizes expression of 112 genes. e, Transcriptional activation of 4,637 genes upon expression MYC(V394D) (MYC(VD)) or wild-type MYC (MYC(WT)) in U2OS cells (r = 0.99; P = 5.1 × 10−26). One-hundred and eighty-six genes are present in each bin.
Extended Data Figure 9 MIZ1 function in genetic models of MYC function.
a, RT–qPCR assays showing the removal of exons encoding the POZ domain upon addition of 4-OHT and induction of MYC (Myc(WT)) or MYC(V394D) (Myc(VD)) in mouse Miz1flox/flox fibroblasts expressing a CreER recombinase. b, Deletion of the Miz1 POZ domain has virtually no effect on expression of MYC-repressed genes in the absence of induced MYC. Data are shown for bins of genes that are upregulated by deletion of the POZ domain in the presence of MYC; x-axis shows 4-OHT-induced change in the presence of doxycycline, y-axis shows change in the absence of doxycycline. c, Restoration of wild-type MIZ1 in mouse Miz1ΔPOZ/ΔPOZ fibroblasts re-establishes MYC-dependent repression. The data are plotted as in Fig. 4b. For each bin, a horizontal line indicates the change in expression upon restoration of MIZ1. P values indicate significance of alterations in gene expression for a given bin. P value, two-tailed paired Student’s t-test. d, Venn diagrams showing overlap in Myc- and Myc/Miz1-binding sites in murine fibroblasts, T-cell lymphoma cells and pancreatic tumour cells. Target gene lists are shown in Supplementary Table 1. e, MYC and Miz1 binding to chromatin in T-cell lymphomas. The Venn diagram illustrates the change in Miz1 DNA binding upon doxycycline-mediated removal of MYC. Of the 12,125 sites that are bound by MYC and Miz1 in T-cell lymphomas, Miz1 remains bound at 5,448 sites in the absence of MYC. f, Venn diagram showing binding of Myc and Miz1 to overlapping binding sites in cells established from pancreatic tumours arising in Pdx1-cre;LSL-KrasG12D/+;LSL-p53R172H/+ mice (P < 2.2 × 10−308).
Supplementary information
Supplementary Table 1
Myc- and Myc/Miz1-bound genes in murine fibroblasts, T-lymphoma cells and pancreatic tumour cells. "+" indicates promoters (-1 kb to +0.5 kb) containing a MACS-called peak (FDR<0.1), "-" indicates promoters without Myc or Myc/Miz1 binding site. (XLS 1373 kb)
Supplementary Table 2
Oligonucleotides used in the study. (XLS 55 kb)
Rights and permissions
About this article
Cite this article
Walz, S., Lorenzin, F., Morton, J. et al. Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature 511, 483–487 (2014). https://doi.org/10.1038/nature13473
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13473
This article is cited by
-
MYC activity at enhancers drives prognostic transcriptional programs through an epigenetic switch
Nature Genetics (2024)
-
Z-ligustilide preferentially caused mitochondrial dysfunction in AML HL-60 cells by activating nuclear receptors NUR77 and NOR1
Chinese Medicine (2023)
-
A competitive precision CRISPR method to identify the fitness effects of transcription factor binding sites
Nature Biotechnology (2023)
-
Targeting MYC with modular synthetic transcriptional repressors derived from bHLH DNA-binding domains
Nature Biotechnology (2023)
-
MYC promotes global transcription in part by controlling P-TEFb complex formation via DNA-binding independent inhibition of CDK9 SUMOylation
Science China Life Sciences (2023)