Abstract
Plasma membrane pannexin 1 channels (PANX1) release nucleotide find-me signals from apoptotic cells to attract phagocytes. Here we show that the quinolone antibiotic trovafloxacin is a novel PANX1 inhibitor, by using a small-molecule screen. Although quinolones are widely used to treat bacterial infections, some quinolones have unexplained side effects, including deaths among children. PANX1 is a direct target of trovafloxacin at drug concentrations seen in human plasma, and its inhibition led to dysregulated fragmentation of apoptotic cells. Genetic loss of PANX1 phenocopied trovafloxacin effects, revealing a non-redundant role for pannexin channels in regulating cellular disassembly during apoptosis. Increase in drug-resistant bacteria worldwide and the dearth of new antibiotics is a major human health challenge. Comparing different quinolone antibiotics suggests that certain structural features may contribute to PANX1 blockade. These data identify a novel linkage between an antibiotic, pannexin channels and cellular integrity, and suggest that re-engineering certain quinolones might help develop newer antibacterials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Penuela, S., Gehi, R. & Laird, D. W. The biochemistry and function of pannexin channels. Biochim. Biophys. Acta 1828, 15–22 (2013)
Sosinsky, G. E. et al. Pannexin channels are not gap junction hemichannels. Channels (Austin) 5, 193–197 (2011)
Chen, Y. et al. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci. Signal. 3, ra45 (2010)
Seminario-Vidal, L. et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J. Biol. Chem. 286, 26277–26286 (2011)
Séror, C. et al. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J. Exp. Med. 208, 1823–1834 (2011)
Billaud, M., Sandilos, J. K. & Isakson, B. E. Pannexin 1 in the regulation of vascular tone. Trends Cardiovasc. Med. 22, 68–72 (2012)
Karatas, H. et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 339, 1092–1095 (2013)
Kim, J. E. & Kang, T. C. The P2X7 receptor-pannexin-1 complex decreases muscarinic acetylcholine receptor-mediated seizure susceptibility in mice. J. Clin. Invest. 121, 2037–2047 (2011)
MacVicar, B. A. & Thompson, R. J. Non-junction functions of pannexin-1 channels. Trends Neurosci. 33, 93–102 (2010)
Lazarowski, E. R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 8, 359–373 (2012)
Dubyak, G. R. Ion homeostasis, channels, and transporters: an update on cellular mechanisms. Adv. Physiol. Educ. 28, 143–154 (2004)
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009)
Chekeni, F. B. et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467, 863–867 (2010)
Sandilos, J. K. et al. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J. Biol. Chem. 287, 11303–11311 (2012)
Qu, Y. et al. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 186, 6553–6561 (2011)
Liu, H. H. Safety profile of the fluoroquinolones: focus on levofloxacin. Drug Saf. 33, 353–369 (2010)
Stahlmann, R. & Lode, H. Safety considerations of fluoroquinolones in the elderly: an update. Drugs Aging 27, 193–209 (2010)
Bruzzone, R., Barbe, M. T., Jakob, N. J. & Monyer, H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J. Neurochem. 92, 1033–1043 (2005)
Ma, W., Hui, H., Pelegrin, P. & Surprenant, A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J. Pharmacol. Exp. Ther. 328, 409–418 (2009)
Vincent, J. et al. Pharmacokinetics and safety of trovafloxacin in healthy male volunteers following administration of single intravenous doses of the prodrug, alatrofloxacin. J. Antimicrob. Chemother. 39 (suppl. B). 75–80 (1997)
Teng, R., Liston, T. E. & Harris, S. C. Multiple-dose pharmacokinetics and safety of trovafloxacin in healthy volunteers. J. Antimicrob. Chemother. 37, 955–963 (1996)
Wickman, G., Julian, L. & Olson, M. F. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 19, 735–742 (2012)
Moss, D. K., Betin, V. M., Malesinski, S. D. & Lane, J. D. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J. Cell Sci. 119, 2362–2374 (2006)
Nagata, S., Hanayama, R. & Kawane, K. Autoimmunity and the clearance of dead cells. Cell 140, 619–630 (2010)
Bortner, C. D. & Cidlowski, J. A. Caspase independent/dependent regulation of K+, cell shrinkage, and mitochondrial membrane potential during lymphocyte apoptosis. J. Biol. Chem. 274, 21953–21962 (1999)
Vanden Berghe, T. et al. Determination of apoptotic and necrotic cell death in vitro and in vivo. Methods 61, 117–129 (2013)
Silverman, W., Locovei, S. & Dahl, G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am. J. Physiol. Cell Physiol. 295, C761–C767 (2008)
Cohen, J. J., Duke, R. C., Fadok, V. A. & Sellins, K. S. Apoptosis and programmed cell death in immunity. Annu. Rev. Immunol. 10, 267–293 (1992)
Baroja-Mazo, A., Barbera-Cremades, M. & Pelegrin, P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim. Biophys. Acta 1828, 79–93 (2013)
Coleman, M. L. et al. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nature Cell Biol. 3, 339–345 (2001)
Sebbagh, M. et al. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nature Cell Biol. 3, 346–352 (2001)
Andriole, V. T. The quinolones: past, present, and future. Clin. Infect. Dis. 41 (suppl. 2). S113–S119 (2005)
Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/ (US Department of Health and Human Services, 2013)
Department of Health. UK five year antimicrobial resistance strategy 2013 to 2018. https://www.gov.uk/government/publications/uk-5-year-antimicrobial-resistance-strategy-2013-to-2018 (2013)
Ahmad, K. Drug company sued over research trial in Nigeria. Lancet 358, 815 (2001)
Spellberg, B. et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46, 155–164 (2008)
Lakso, M. et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl Acad. Sci. USA 93, 5860–5865 (1996)
Lee, P. P. et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001)
Harris, A. L. Connexin channel permeability to cytoplasmic molecules. Prog. Biophys. Mol. Biol. 94, 120–143 (2007)
Henry, C. M., Hollville, E. & Martin, S. J. Measuring apoptosis by microscopy and flow cytometry. Methods 61, 90–97 (2013)
Acknowledgements
We thank B. Isakson, M. Billaud, J. Lannigan and other colleagues for discussions. Grants from the US National Institutes of Health (NIGMS 107848 to K.S.R. and D.B.) and the National Health & Medical Research Council of Australia (I.K.H.P.), a training fellowship to I.K.H.P., and the American Heart Association (J.M.K.) supported this work.
Author information
Authors and Affiliations
Contributions
I.K.H.P. designed, performed and analysed most of the experiments with input from K.S.R. Y.-H.C. performed and analysed the patch-clamp studies with input from D.A.B. A.J.A. generated the PANX1 knockout mice and Jurkat cell lines expressing PANX1. J.M.K. helped with the drug screen and microscopy experiments. I.J.J. performed primary T-cell isolation and quantitative PCR. A.J.A. and I.J.J. assisted with the initial characterization of PANX1-deficient mice. I.K.H.P. and K.S.R. wrote the manuscript with input from co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
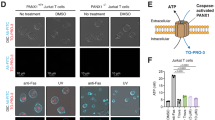
Extended Data Figure 1 Trovafloxacin does not block caspase activation or inhibit connexin 43 (Cx43) or pannexin 2 (Panx2) membrane currents.
a, Caspase 3/7 activation in Jurkat cells undergoing apoptosis is not altered by treatment with trovafloxacin (40 μM) (n = 3). b, Proteolytic cleavage of PANX1–GFP during apoptosis is not inhibited by trovafloxacin (40 µM) or CBX (500 μM) treatment. c, Schematic diagram for the acute treatment of apoptotic cells with trovafloxacin or CBX. d, Acute trovafloxacin treatment inhibits TO-PRO-3 uptake by apoptotic Jurkat cells. Left, histograms showing TO-PRO-3 uptake by viable cells, apoptotic cells, or apoptotic cells treated with trovafloxacin or CBX (500 μM) post induction of apoptosis and analysed by flow cytometry. Right, uptake of TO-PRO-3 presented as (median fluorescence intensity, MFI) of viable cells or apoptotic cells (n = 3). e, Inhibition of CBX-sensitive current in apoptotic cells treated with trovafloxacin (20 μM), as measured by whole-cell patch-clamp recording (n = 7). f, Patch-clamp recordings from HEK293T cells expressing Cx43 and receiving indicated treatments. Whole-cell current at +80 mV is shown under conditions when bath solution was perfused with trovafloxacin (20 μM, blue shading) or gadolinium (Gd3+) (100 μM, pink shading). g, Current-voltage relationships of Cx43 current in HEK293T cells treated with or without trovafloxacin (20 μM) or Gd3+ (100 μM), with the current measured over a range of voltages. Exemplar traces in f and g are representative of 14 cells per group. h, Patch-clamp recordings from HEK293T cells expressing mouse Panx2 and receiving indicated treatments. Whole-cell current at +80 mV is shown under conditions when bath solution was perfused with trovafloxacin (20 μM, blue shading) or carbenoxolone (CBX) (50 μM, pink shading). i, Current-voltage relationships of Panx2 current in HEK293T cells treat with or without trovafloxacin (20 μM) or CBX (50 μM), with the current measured over a range of voltages. Exemplar traces in h and i are representative of 4 cells per group. j, Trovafloxacin, ciprofloxacin and levofloxacin inhibit bacterial growth. Escherichia coli growth (as measured by absorbance at 600 nm) in the presence of indicated concentrations of quinolones (n = 3). Error bars represent s.e.m.
Extended Data Figure 2 Electronic gating strategy for the separation of different cellular and subcellular population of Jurkat cells undergoing apoptosis in vitro.
a, Flow cytometric analysis showing each type of particles gated (see b below) has a distinctive level of cellular complexity (side scatter, SSC), cell size (forward scatter, FSC) as well as TO-PRO-3 (indicative of caspase-mediated activation of pannexin 1 channels), 7-AAD (indicative of membrane integrity) and annexin V (indicative of phosphatidylserine exposure) staining. b, Flow cytometry gating strategy used to distinguish viable cells, annexin V− apoptotic cells, annexin V+ apoptotic cells, annexin V− particles, and apoptotic bodies. c, ImageStream analysis of particles gated using the same strategy as described in b. Representative images for each type of particles are shown. Jurkat cells were induced to undergo apoptosis by anti-Fas treatment (2 h) in all indicated experiments.
Extended Data Figure 3 Inhibition of pannexin 1 promotes the formation of apoptotic bodies via a mechanism independent of extracellular ATP.
a, CBX and probenecid enhance the generation of apoptotic bodies from cells undergoing ultraviolet-induced apoptosis (n = 3). b, Formation of apoptotic bodies after treatment with the indicated concentrations of CBX (n = 3). The corresponding TO-PRO-3 uptake by annexin V+ apoptotic cells at each CBX concentration is shown above the respective bars. c, d, Addition of exogenous ATP during apoptosis induction does not inhibit formation of apoptotic bodies in CBX-treated cells (n = 3) (c) or cells stably expressing the dominant-negative PANX1 mutant (PANX1 DN mutant) (n = 3) (d). e, Removal of extracellular ATP by apyrase does not promote formation of apoptotic bodies (n = 3). f, P2Y receptor antagonist suramin does not promote formation of apoptotic bodies (n = 3). Jurkat cells were induced to undergo apoptosis by anti-Fas treatment in all indicated experiments. Error bars represent s.e.m.
Extended Data Figure 4 Pannexin 1 activity does not affect DNA fragmentation during apoptosis.
a, b, TO-PRO-3 dye uptake (n = 3) (a) and DNA fragmentation (b) were assessed in Jurkat cells stably expressing the control vector, the dominant-negative PANX1 mutant (PANX1 DN mutant) or wild-type PANX1 (PANX1 WT). DNA fragmentation from cells induced to undergo apoptosis and treated with or without 500 μM CBX is also shown in b. c, Time-lapse images monitoring TO-PRO-3 dye uptake during progression of apoptosis in Jurkat cells with normal PANX1 function show that TO-PRO-3 uptake occurs before initiation of membrane blebbing. Jurkat cells were induced to undergo apoptosis by anti-Fas treatment (2 h). Error bars in a represent s.e.m.
Extended Data Figure 5 Electronic gating strategy for the separation of different cellular and subcellular populations of primary thymocytes undergoing apoptosis ex vivo.
a, Flow cytometry analysis showing each type of particle gated according to b has a distinctive level of SSC, FSC as well as TO-PRO-3 and annexin V staining. b, Flow cytometry analysis showing electronic gating strategy used to distinguish viable cells, annexin V− apoptotic cells, annexin V+ apoptotic cells, annexin V− particles, and apoptotic bodies. c, ImageStream analysis of particles gated using the same strategy as described in b. Representative images for each type of particle are shown. Primary mouse thymocytes were induced to undergo apoptosis by dexamethasone (Dex) treatment in all indicated experiments.
Extended Data Figure 6 Electronic gating strategy for analysing the complexity of subcellular apoptotic particles generated ex vivo and in vivo.
a, Flow cytometry analysis showing electronic gating strategy used to distinguish annexin Vhigh, 7-AADlow subcellular particles generated from primary mouse thymocytes induced to undergo apoptosis via dexamethasone treatment. Subcellular apoptotic particles with high complexity (SSC high) or low complexity (SSC low) are gated as shown. b, Flow cytometry analysis showing electronic gating strategy used to distinguish different subsets of apoptotic cell-derived particles generated in the thymus of mice injected intraperitoneally with dexamethasone (6 h). Annexin Vhigh, 7-AADlow, CD4/CD8intermediate particles were initially selected and subsequently gated based on forward scatter (FSC, indicative of cell size). Apoptotic particles of interest (as indicated) are therefore defined as annexin Vhigh, 7-AADlow, CD4/CD8intermediate and FSClow/intermediate.
Extended Data Figure 7 Generation of conditional and global pannexin 1-deficient mice.
a, Strategy for deletion of neomycin cassette and exon 3 of Panx1. b, Identification of mice with floxed Panx1 loci, assessed by PCR. c, mRNA levels of Panx1 in CD4+ thymocytes relative to Gapdh. n = 3 mice per group. d, Immunoblotting of lysates from thymocytes with the indicated genotypes. e, Identification of mice with wild-type, heterozygous and homozygous Panx1-targeted loci, assessed by PCR. f, mRNA levels of Panx1 in thymocytes with the indicated genotypes relative to Gapdh. n = 3 mice per group. Error bars in c and f represent s.e.m.
Extended Data Figure 8 Formation of apoptotic bodies but not string-like apoptopodia structures is dependent on actomyosin contraction.
a, Time-lapse images monitoring apoptotic cell morphology of cells treated with or without CBX (500 μM) and in the presence of actomyosin contraction inhibitors. Top right, percentage of apoptotic cells forming string-like apoptopodia structures (387, 414, 459 and 372 apoptotic cells were analysed for Y-27632, Y-27632+CBX, Cyto-D and Cyto-D+CBX-treated cells, respectively, from three independent experiments). b, c, Time-lapse images monitoring apoptotic cell morphology of cells stably expressing the dominant-negative PANX1 mutant (PANX DN mutant) (b) or treated with 40 μM trovafloxacin (c) in the presence of Cyto-D (5 μM). d, Inhibitors of blebbing, Y-27632, blebbistatin, or cytochalasin D (Cyto-D) reduce the formation of apoptotic bodies in Jurkat cells expressing PANX1 DN mutant (n = 3). e, Generation of apoptotic bodies by dying cells treated with Y-27632 (10 μM), blebbistatin (50 μM) and Cyto-D (5 μM). Cells were induced to undergo apoptosis in the presence or absence of CBX (500 μM) (n = 3). f, The enhanced formation of apoptotic bodies in apoptotic thymocytes from mice with PANX1 deficiency is also blunted by the ROCK inhibitor Y-27632 (10 μM) that blocks membrane blebbing (n = 3). Error bars represent s.e.m. Scale bars, 5 μm. Arrows, apoptopodia.
Extended Data Figure 9 Inhibition of pannexin 1 during ultraviolet-induced apoptosis in LR73 fibroblasts promotes the formation of membrane protrusions and apoptotic bodies.
a, ATP levels in supernatants of LR73 fibroblasts treated with 40 μM trovafloxacin with or without apoptosis induction (n = 3). b, Formation of apoptotic bodies (left) and TO-PRO-3 uptake (right) by LR73 fibroblasts treated with the indicated concentrations of trovafloxacin (n = 3). c, Generation of apoptotic bodies by LR73 fibroblasts treated with 2 mM probenecid (n = 3). d, Time-lapse images monitoring apoptotic cell morphology of LR73 fibroblasts treated with or without trovafloxacin (40 μM) or probenecid (2 mM). LR73 fibroblasts were induced to undergo apoptosis by ultraviolet treatment in all indicated experiments. Error bars in a–c represent s.e.m. Arrows, apoptopodia. Scale bars, 10 μm.
Extended Data Figure 10 Schematic diagram depicting where pannexin 1 likely acts in limiting the fragmentation of apoptotic cells.
Blocking PANX1 function (for example via trovafloxacin) leads to formation of apoptopodia, and subsequently the release of apoptotic bodies.
Supplementary information
Jurkat cells induced to undergo Fas-mediated apoptosis
Time-lapse differential interface contrast video monitoring apoptotic cell morphology of Jurkat cells. Scale bar represent 5 µm. (MP4 8994 kb)
Jurkat cells induced to undergo Fas-mediated apoptosis in the presence of trovafloxacin
Time-lapse differential interface contrast video monitoring apoptotic cell morphology of Jurkat cells treated with 40 µM trovafloxacin. Scale bar represent 5 µm. (MP4 5775 kb)
Jurkat cells induced to undergo Fas-mediated apoptosis in the presence of carbenoxolone
Time-lapse differential interface contrast video monitoring apoptotic cell morphology of Jurkat cells treated with 500 µM carbenoxolone. Scale bar represent 5 µm. (MP4 3746 kb)
Rights and permissions
About this article
Cite this article
Poon, I., Chiu, YH., Armstrong, A. et al. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 507, 329–334 (2014). https://doi.org/10.1038/nature13147
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13147