Abstract
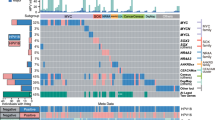
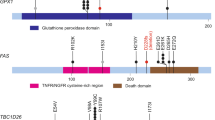
Cervical cancer is responsible for 10–15% of cancer-related deaths in women worldwide1,2. The aetiological role of infection with high-risk human papilloma viruses (HPVs) in cervical carcinomas is well established3. Previous studies have also implicated somatic mutations in PIK3CA, PTEN, TP53, STK11 and KRAS4,5,6,7 as well as several copy-number alterations in the pathogenesis of cervical carcinomas8,9. Here we report whole-exome sequencing analysis of 115 cervical carcinoma–normal paired samples, transcriptome sequencing of 79 cases and whole-genome sequencing of 14 tumour–normal pairs. Previously unknown somatic mutations in 79 primary squamous cell carcinomas include recurrent E322K substitutions in the MAPK1 gene (8%), inactivating mutations in the HLA-B gene (9%), and mutations in EP300 (16%), FBXW7 (15%), NFE2L2 (4%), TP53 (5%) and ERBB2 (6%). We also observe somatic ELF3 (13%) and CBFB (8%) mutations in 24 adenocarcinomas. Squamous cell carcinomas have higher frequencies of somatic nucleotide substitutions occurring at cytosines preceded by thymines (Tp*C sites) than adenocarcinomas. Gene expression levels at HPV integration sites were statistically significantly higher in tumours with HPV integration compared with expression of the same genes in tumours without viral integration at the same site. These data demonstrate several recurrent genomic alterations in cervical carcinomas that suggest new strategies to combat this disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Data deposits
Sequence data used for this analysis are available in dbGaP under accession phs000600.
References
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011)
International Agency for Research on Cancer. A review of human carcinogen: biological agents. in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 100B (International Agency for Research on Cancer, 2012)
zur Hausen, H. Papillomaviruses in the causation of human cancers — a brief historical account. Virology 384, 260–265 (2009)
Crook, T. et al. Clonal p53 mutation in primary cervical cancer: association with human-papillomavirus-negative tumours. Lancet 339, 1070–1073 (1992)
McIntyre, J. B. et al. PIK3CA mutational status and overall survival in patients with cervical cancer treated with radical chemoradiotherapy. Gynecol. Oncol. 128, 409–414 (2013)
Kang, S. et al. Inverse correlation between RASSF1A hypermethylation, KRAS and BRAF mutations in cervical adenocarcinoma. Gynecol. Oncol. 105, 662–666 (2007)
Wingo, S. N. et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS ONE 4, e5137 (2009)
Narayan, G. & Murty, V. V. Integrative genomic approaches in cervical cancer: implications for molecular pathogenesis. Future Oncol. 6, 1643–1652 (2010)
Vazquez-Mena, O. et al. Amplified genes may be overexpressed, unchanged, or downregulated in cervical cancer cell lines. PLoS ONE 7, e32667 (2012)
Arteaga, C. L. & Baselga, J. Impact of genomics on personalized cancer medicine. Clin. Cancer Res. 18, 612–618 (2012)
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature Biotechnol. 31, 213–219 (2013)
Lawrence, M. S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013)
Lohr, J. G. et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc. Natl Acad. Sci. USA 109, 3879–3884 (2012)
Greulich, H. et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc. Natl Acad. Sci. USA 109, 14476–14481 (2012)
Bose, R. et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 3, 224–237 (2012)
Arvind, R. et al. A mutation in the common docking domain of ERK2 in a human cancer cell line, which was associated with its constitutive phosphorylation. Int. J. Oncol. 27, 1499–1504 (2005)
De Luca, A., Maiello, M. R., D’Alessio, A., Pergameno, M. & Normanno, N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin. Ther. Targets 16 (Suppl. 2). S17–S27 (2012)
Le Gallo, M. et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nature Genet. 44, 1310–1315 (2012)
Agrawal, N. et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333, 1154–1157 (2011)
Chen, J., Ghazawi, F. M. & Li, Q. Interplay of bromodomain and histone acetylation in the regulation of p300-dependent genes. Epigenetics 5, 509–515 (2010)
Smith, T. F., Gaitatzes, C., Saxena, K. & Neer, E. J. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24, 181–185 (1999)
Tong, K. I. et al. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol. Cell. Biol. 26, 2887–2900 (2006)
Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012)
Pamer, E. & Cresswell, P. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 16, 323–358 (1998)
Neve, R. M., Ylstra, B., Chang, C. H., Albertson, D. G. & Benz, C. C. ErbB2 activation of ESX gene expression. Oncogene 21, 3934–3938 (2002)
Wentzensen, N., Vinokurova, S. & von Knebel Doeberitz, M. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 64, 3878–3884 (2004)
Kraus, I. et al. The majority of viral-cellular fusion transcripts in cervical carcinomas cotranscribe cellular sequences of known or predicted genes. Cancer Res. 68, 2514–2522 (2008)
Schmitz, M., Driesch, C., Jansen, L., Runnebaum, I. B. & Durst, M. Non-random integration of the HPV genome in cervical cancer. PLoS ONE 7, e39632 (2012)
Tang, K. W., Alaei-Mahabadi, B., Samuelsson, T., Lindh, M. & Larsson, E. The landscape of viral expression and host gene fusion and adaptation in human cancer. Nature Commun. 4, 2513 (2013)
Peter, M. et al. Frequent genomic structural alterations at HPV insertion sites in cervical carcinoma. J. Pathol. 221, 320–330 (2010)
Gnirke, A. et al. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nature Biotechnol. 27, 182–189 (2009)
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009)
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009)
Stransky, N. et al. The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160 (2011)
Banerji, S. et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 486, 405–409 (2012)
Lee, R. S. et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J. Clin. Invest. 122, 2983–2988 (2012)
Berger, M. F. et al. The genomic complexity of primary human prostate cancer. Nature 470, 214–220 (2011)
Chapman, M. A. et al. Initial genome sequencing and analysis of multiple myeloma. Nature 471, 467–472 (2011)
Cibulskis, K. et al. ContEst: estimating cross-contamination of human samples in next-generation sequencing data. Bioinformatics 27, 2601–2602 (2011)
Forbes, S. A. et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 39, D945–D950 (2011)
DePristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genet. 43, 491–498 (2011)
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010)
Carter, S. L. et al. Absolute quantification of somatic DNA alterations in human cancer. Nature Biotechnol. 30, 413–421 (2012)
Erlich, H. HLA DNA typing: past, present, and future. Tissue Antigens 80, 1–11 (2012)
Ward, J. H., Jr Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 58, 236–244 (1963)
Trapnell, C. et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nature Biotechnol. 31, 46–63 (2012)
Bass, A. J. et al. Genomic sequencing of colorectal adenocarcinomas identifies a recurrent VTI1A-TCF7L2 fusion. Nature Genet. 43, 964–968 (2011)
Pleasance, E. D. et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463, 191–196 (2010)
Wilkerson, M. D. & Hayes, D. N. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics 26, 1572–1573 (2010)
Cañadas, M. P. et al. Comparison of the f-HPV typing and Hybrid Capture II assays for detection of high-risk HPV genotypes in cervical samples. J. Virol. Methods 183, 14–18 (2012)
Walline, H. M. et al. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and, oral cavity cancers: comparison of multiple methods. JAMA Otolaryngol. Head Neck Surg. http://dx.doi.org/10.1001/jamaoto.2013.5460. (31 October 2013)
Yang, H. et al. Sensitive detection of human papillomavirus in cervical, head/neck, and schistosomiasis-associated bladder malignancies. Proc. Natl Acad. Sci. USA 102, 7683–7688 (2005)
Kostic, A. D. et al. PathSeq: software to identify or discover microbes by deep sequencing of human tissue. Nature Biotechnol. 29, 393–396 (2011)
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005)
Harris, R. S. & Liddament, M. T. Retroviral restriction by APOBEC proteins. Nature Rev. Immunol. 4, 868–877 (2004)
Roberts, S. A. et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nature Genet. 45, 970–976 (2013)
Acknowledgements
This work was conducted as part of the Slim Initiative for Genomic Medicine in the Americas, a project funded by the Carlos Slim Health Institute in Mexico. This work was also partially supported by the Rebecca Ridley Kry Fellowship of the Damon Runyon Cancer Research Foundation (A.I.O.); MMRF Research Fellow Award (A.I.O.); Helse Vest, Research Council of Norway, Norwegian Cancer Society and Harald Andersens legat (H.B.S.); CONACyT grant SALUD-2008-C01-87625 and UANL PAICyT grant CS1038-1 (H.A.B.-S.); and CONACyT grant 161619 (J.M.-Z.). We also thank B. Edvardsen, K. Dahl-Michelsen, Å. Mokleiv, K. Madisso, T. Njølstad and E. Valen for technical and programmatic assistance; the staff of the Broad Institute Genomics Platform for their assistance in processing samples and generating the sequencing data used in the analyses; the Instituto Mexicano del Seguro Social (IMSS) for their Support; and L. Gaffney of Broad Institute Communications for figure layout and design.
Author information
Authors and Affiliations
Contributions
A.I.O., L.L., S.S.F., C.S.P., H.B.S. and M.M. wrote the manuscript with help from co-authors. A.I.O., L.L., K.C., C.S. and G.G. performed whole exome and genome sequencing data analysis. A.I.O., I.I., V.T., K.V.-S., A.S.G., S.R.-C., C.R.E., S.S.F. and C.S.P. performed RNA sequencing data analysis. A.I.O., S.S.F., C.S.P. and T.J.P. performed HPV integration analyses. A.I.O. and A.D.C. performed copy-number analyses. A.I.O., F.D., B.K., R.W. and H.G. performed functional experiments on MAPK1. B.B., N.B.G., G.S.G.-M. and C.P.C. facilitated and performed pathology review. O.K.V., H.M.W. and T.E.C. performed HPV status determination. L.A., E.N. and M.L.C. facilitated project management. L.L., I.I.-R., V.T., K.V.-S., A.S.G., S.R.-C., I.P.R.-S. and C.R.E. performed sequencing data validation. M.E.-C., M.K.H., E.W., E.A.H., C.K. and M.L.G.-R. performed specimen processing, biobanking and data management. K.W., L.B., L.D.V.-C., G.M., J.V., C.R., A.C. and H.B.S. collected patient materials and clinical information. A.I.O., L.L. and D.S.N. performed biostatistical and epidemiological analyses. A.I.O., L.L., S.S.F., C.S.P., I.I.-R., T.J.P., A.D.C., V.T., A.A.W., M.W.R., F.D., M.S.L., C.S., S.L.C., A.M., H.B.S. and M.M. contributed text, figures (including Supplementary Information) and analytical tools. A.H.-M., C.R.E., L.A.A., S.B.G., H.A.B.-S., J.M.-Z., G.G., H.B.S. and M.M. provided leadership for the project. All authors contributed to the final manuscript. Lead authors A.I.O. and L.L. and senior authors M.M. and H.B.S. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
M.M. holds equity in, and consults for, Foundation Medicine.
Supplementary information
Supplementary Information
This file contains Supplementary Notes 1-15 with additional references (see Contents for more details), Supplementary Figures 1-30 and Supplementary Tables 1-11, 13 and 15-21 (see separate files for tables 12 and 14). (PDF 5727 kb)
Supplementary Table 12
This zipped file contains the correlation between RNASeq-derived gene expression and WES-derived copy number across 16898 genes, as well as the full complement of the raw values for these two parameters for 79 tumors with RNASeq data. (ZIP 30025 kb)
Supplementary Table 14
This file contains details of HPV typing and viral integration analyses. (XLSX 56 kb)
Rights and permissions
About this article
Cite this article
Ojesina, A., Lichtenstein, L., Freeman, S. et al. Landscape of genomic alterations in cervical carcinomas. Nature 506, 371–375 (2014). https://doi.org/10.1038/nature12881
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12881