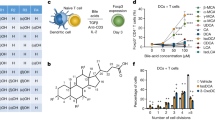
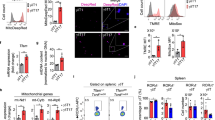
Abstract
Intestinal microbes provide multicellular hosts with nutrients and confer resistance to infection. The delicate balance between pro- and anti-inflammatory mechanisms, essential for gut immune homeostasis, is affected by the composition of the commensal microbial community. Regulatory T cells (Treg cells) expressing transcription factor Foxp3 have a key role in limiting inflammatory responses in the intestine1. Although specific members of the commensal microbial community have been found to potentiate the generation of anti-inflammatory Treg or pro-inflammatory T helper 17 (TH17) cells2,3,4,5,6, the molecular cues driving this process remain elusive. Considering the vital metabolic function afforded by commensal microorganisms, we reasoned that their metabolic by-products are sensed by cells of the immune system and affect the balance between pro- and anti-inflammatory cells. We tested this hypothesis by exploring the effect of microbial metabolites on the generation of anti-inflammatory Treg cells. We found that in mice a short-chain fatty acid (SCFA), butyrate, produced by commensal microorganisms during starch fermentation, facilitated extrathymic generation of Treg cells. A boost in Treg-cell numbers after provision of butyrate was due to potentiation of extrathymic differentiation of Treg cells, as the observed phenomenon was dependent on intronic enhancer CNS1 (conserved non-coding sequence 1), essential for extrathymic but dispensable for thymic Treg-cell differentiation1,7. In addition to butyrate, de novo Treg-cell generation in the periphery was potentiated by propionate, another SCFA of microbial origin capable of histone deacetylase (HDAC) inhibition, but not acetate, which lacks this HDAC-inhibitory activity. Our results suggest that bacterial metabolites mediate communication between the commensal microbiota and the immune system, affecting the balance between pro- and anti-inflammatory mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Josefowicz, S. Z. et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399 (2012)
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010)
Ivanov, I. I. et al. Induction of intestinal TH17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009)
Lathrop, S. K. et al. Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254 (2011)
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous clostridium species. Science 331, 337–341 (2011)
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013)
Zheng, Y. et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463, 808–812 (2010)
Annison, G., Illman, R. J. & Topping, D. L. Acetylated, propionylated or butyrylated starches raise large bowel short-chain fatty acids preferentially when fed to rats. J. Nutr. 133, 3523–3528 (2003)
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013)
van Loosdregt, J. et al. Rapid temporal control of Foxp3 protein degradation by sirtuin-1. PLoS ONE 6, e19047 (2011)
van Loosdregt, J. et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood 115, 965–974 (2010)
Zhang, H., Xiao, Y., Zhu, Z., Li, B. & Greene, M. I. Immune regulation by histone deacetylases: a focus on the alteration of FOXP3 activity. Immunol. Cell Biol. 90, 95–100 (2012)
Song, X. et al. Structural and biological features of FOXP3 dimerization relevant to regulatory T cell function. Cell Rep. 1, 665–675 (2012)
Wang, L., de Zoeten, E. F., Greene, M. I. & Hancock, W. W. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nature Rev. Drug Discov. 8, 969–981 (2009)
Tao, R. et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nature Med. 13, 1299–1307 (2007)
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009)
Thangaraju, M. et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 69, 2826–2832 (2009)
Nilsson, N. E., Kotarsky, K., Owman, C. & Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 303, 1047–1052 (2003)
MacDonald, K. P. et al. Effector and regulatory T-cell function is differentially regulated by RelB within antigen-presenting cells during GVHD. Blood 109, 5049–5057 (2007)
Zhu, H. C. et al. Tolerogenic dendritic cells generated by RelB silencing using shRNA prevent acute rejection. Cell. Immunol. 274, 12–18 (2012)
Shih, V. F. et al. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-κB pathways. Nature Immunol. 13, 1162–1170 (2012)
Fontenot, J. D. et al. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 22, 329–341 (2005)
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature Immunol. 8, 191–197 (2007)
Torii, T. et al. Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: specimen stability. Ann. Clin. Biochem. 47, 447–452 (2010)
Samstein, R. M. et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell 151, 153–166 (2012)
Acknowledgements
This work was supported by the Robert Black Fellowship of the Damon Runyon Cancer Research Foundation DRG-2143-13 (N.A.), Ludwig Center at Memorial Sloan Kettering Cancer Center and the US National Institutes of Health (NIH) grant T32 A1007621 (N.A.) and R37 AI034206 (A.Y.R.). A.Y.R. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
N.A., C.C. and X.F. performed experiments and analysed data, with general assistance from S.D. and assistance from P.d. for HPLC, P.J.C. for immunoprecipitation of acetylated Foxp3 and J.v.d.V. for ChIP–qPCR experiments. H.L. and J.R.C. performed LC–MS. N.A., C.C., X.F. and A.Y.R. designed and interpreted experiments. K.P. provided Gpr109a mice. N.A. and A.Y.R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-6. (PDF 407 kb)
Rights and permissions
About this article
Cite this article
Arpaia, N., Campbell, C., Fan, X. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013). https://doi.org/10.1038/nature12726
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12726
This article is cited by
-
Intratumoral microorganisms in tumors of the digestive system
Cell Communication and Signaling (2024)
-
Association of gut microbiota with the pathogenesis of SARS-CoV-2 Infection in people living with HIV
BMC Microbiology (2024)
-
Dietary novel alkaline protease from Bacillus licheniformis improves broiler meat nutritional value and modulates intestinal microbiota and metabolites
Animal Microbiome (2024)
-
Microbes little helpers and suppliers for therapeutic asthma approaches
Respiratory Research (2024)
-
The gut-liver axis in hepatobiliary diseases
Inflammation and Regeneration (2024)