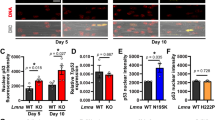
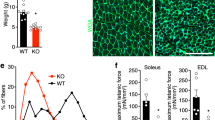
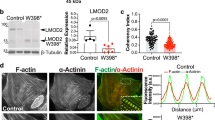
Abstract
Laminopathies, caused by mutations in the LMNA gene encoding the nuclear envelope proteins lamins A and C, represent a diverse group of diseases that include Emery–Dreifuss muscular dystrophy (EDMD), dilated cardiomyopathy (DCM), limb-girdle muscular dystrophy, and Hutchison–Gilford progeria syndrome1. Most LMNA mutations affect skeletal and cardiac muscle by mechanisms that remain incompletely understood. Loss of structural function and altered interaction of mutant lamins with (tissue-specific) transcription factors have been proposed to explain the tissue-specific phenotypes1. Here we report in mice that lamin-A/C-deficient (Lmna−/−) and LmnaN195K/N195K mutant cells have impaired nuclear translocation and downstream signalling of the mechanosensitive transcription factor megakaryoblastic leukaemia 1 (MKL1), a myocardin family member that is pivotal in cardiac development and function2. Altered nucleo-cytoplasmic shuttling of MKL1 was caused by altered actin dynamics in Lmna−/− and LmnaN195K/N195K mutant cells. Ectopic expression of the nuclear envelope protein emerin, which is mislocalized in Lmna mutant cells and also linked to EDMD and DCM, restored MKL1 nuclear translocation and rescued actin dynamics in mutant cells. These findings present a novel mechanism that could provide insight into the disease aetiology for the cardiac phenotype in many laminopathies, whereby lamin A/C and emerin regulate gene expression through modulation of nuclear and cytoskeletal actin polymerization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ho, C. Y. & Lammerding, J. Lamins at a glance. J. Cell Sci. 125, 2087–2093 (2012)
Olson, E. N. & Nordheim, A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nature Rev. Mol. Cell Biol. 11, 353–365 (2010)
Parmacek, M. S. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ. Res. 100, 633–644 (2007)
Miralles, F., Posern, G., Zaromytidou, A. I. & Treisman, R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329–342 (2003)
Mouilleron, S., Guettler, S., Langer, C. A., Treisman, R. & McDonald, N. Q. Molecular basis for G-actin binding to RPEL motifs from the serum response factor coactivator MAL. EMBO J. 27, 3198–3208 (2008)
Hirano, H. & Matsuura, Y. Sensing actin dynamics: structural basis for G-actin-sensitive nuclear import of MAL. Biochem. Biophys. Res. Commun. 414, 373–378 (2011)
Pawłowski, R., Rajakyla, E. K., Vartiainen, M. K. & Treisman, R. An actin-regulated importin α/β-dependent extended bipartite NLS directs nuclear import of MRTF-A. EMBO J. 29, 3448–3458 (2010)
Vartiainen, M. K., Guettler, S., Larijani, B. & Treisman, R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749–1752 (2007)
Lammerding, J. et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 113 370–378 10.1172/JCI19670 (2004)
Cupesi, M. et al. Attenuated hypertrophic response to pressure overload in a lamin A/C haploinsufficiency mouse. J. Mol. Cell. Cardiol. 48, 1290–1297 (2010)
Mounkes, L. C., Kozlov, S. V., Rottman, J. N. & Stewart, C. L. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum. Mol. Genet. 14, 2167–2180 (2005)
Guettler, S., Vartiainen, M. K., Miralles, F., Larijani, B. & Treisman, R. RPEL motifs link the serum response factor cofactor MAL but not myocardin to Rho signaling via actin binding. Mol. Cell. Biol. 28, 732–742 (2008)
Fairley, E. A., Kendrick-Jones, J. & Ellis, J. A. The Emery-Dreifuss muscular dystrophy phenotype arises from aberrant targeting and binding of emerin at the inner nuclear membrane. J. Cell Sci. 112, 2571–2582 (1999)
Holaska, J. M., Kowalski, A. K. & Wilson, K. L. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol. 2, e231 (2004)
Nikolova-Krstevski, V. et al. Nesprin-1 and actin contribute to nuclear and cytoskeletal defects in lamin A/C-deficient cardiomyopathy. J. Mol. Cell. Cardiol. 50, 479–486 (2011)
Hale, C. M. et al. Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys. J. 95, 5462–5475 (2008)
Salpingidou, G., Smertenko, A., Hausmanowa-Petrucewicz, I., Hussey, P. J. & Hutchison, C. J. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J. Cell Biol. 178, 897–904 (2007)
Simon, D. N., Zastrow, M. S. & Wilson, K. L. Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus 1, 264–272 (2010)
Wilson, K. L. & Berk, J. M. The nuclear envelope at a glance. J. Cell Sci. 123, 1973–1978 (2010)
Muehlich, S. et al. Serum-induced phosphorylation of the serum response factor coactivator MKL1 by the extracellular signal-regulated kinase 1/2 pathway inhibits its nuclear localization. Mol. Cell. Biol. 28, 6302–6313 (2008)
Nikolova, V. et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J. Clin. Invest. 113, 357–369 (2004)
Morita, T., Mayanagi, T. & Sobue, K. Reorganization of the actin cytoskeleton via transcriptional regulation of cytoskeletal/focal adhesion genes by myocardin-related transcription factors (MRTFs/MAL/MKLs). Exp. Cell Res. 313, 3432–3445 (2007)
Parlakian, A. et al. Temporally controlled onset of dilated cardiomyopathy through disruption of the SRF gene in adult heart. Circulation 112, 2930–2939 (2005)
Lammerding, J. et al. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J. Cell Biol. 170, 781–791 (2005)
Rowat, A. C., Lammerding, J. & Ipsen, J. H. Mechanical properties of the cell nucleus and the effect of emerin deficiency. Biophys. J. 91, 4649–4664 (2006)
Melcon, G. et al. Loss of emerin at the nuclear envelope disrupts the Rb1/E2F and MyoD pathways during muscle regeneration. Hum. Mol. Genet. 15, 637–651 (2006)
Mokalled, M. H., Johnson, A. N., Creemers, E. E. & Olson, E. N. MASTR directs MyoD-dependent satellite cell differentiation during skeletal muscle regeneration. Genes Dev. 26, 190–202 (2012)
Muchir, A., Shan, J., Bonne, G., Lehnart, S. E. & Worman, H. J. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum. Mol. Genet. 18, 241–247 (2009)
Sullivan, T. et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147, 913–920 (1999)
Kudo, N. et al. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 242, 540–547 (1998)
Knowles, G. C. & McCulloch, C. A. Simultaneous localization and quantification of relative G and F actin content: optimization of fluorescence labeling methods. J. Histochem. Cytochem. 40, 1605–1612 (1992)
Phair, R. D. & Misteli, T. High mobility of proteins in the mammalian cell nucleus. Nature 404, 604–609 (2000)
McDonald, D., Carrero, G., Andrin, C., de Vries, G. & Hendzel, M. J. Nucleoplasmic beta-actin exists in a dynamic equilibrium between low-mobility polymeric species and rapidly diffusing populations. J. Cell Biol. 172, 541–552 (2006)
Acknowledgements
We thank C. Stewart for the mouse models and cell lines. We thank J. Gannon for TAC surgeries and M. Cupesi for collecting the cardiac samples from the pressure-overload model. This work was supported by National Institutes of Health awards (R01 NS059348 and R01 HL082792); the Department of Defense Breast Cancer Idea Award (BC102152); an award from the Progeria Research Foundation (PRF 2011-035); and a postdoctoral fellowship from the American Heart Association to D.E.J. (AHA award 09POST2320042). The work in the laboratory of M.K.V. is funded by the Academy of Finland and the Sigrid Juselius Foundation.
Author information
Authors and Affiliations
Contributions
C.Y.H., D.E.J. and J.L. conceived and designed the overall project, with valuable help from M.K.V. C.Y.H. and D.E.J. performed the experiments. C.Y.H., D.E.J. and J.L. analysed data. C.Y.H. and J.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10 and Supplementary References. (PDF 2022 kb)
Nuclear translocation of MKL1-GFP in Lmna+/+ MEFs
This video shows an Lmna+/+ mouse embryonic fibroblast expressing MKL1-GFP imaged before (frame 1) and immediately after serum stimulation (frames 2 and onwards). This time lapse covers a period of about 20 minutes. MKL1-GFP accumulated in the nucleus during the course of the video. (MOV 169 kb)
Nuclear translocation of MKL1-GFP in Lmna–/– MEFs
This video shows an Lmna–/– mouse embryonic fibroblast expressing MKL1-GFP imaged before (frame 1) and immediately after serum stimulation (frames 2 and onwards). This time lapse covers a period of about 20 minutes. Nuclear accumulation of MKL1-GFP is not evident during the course of the video. (MOV 428 kb)
Nuclear translocation of MKL1-GFP in Lmna N195K MEFs
This video shows an Lmna N195K mouse embryonic fibroblast expressing MKL1-GFP imaged before (frame 1) and immediately after serum stimulation (frames 2 and onwards). This time lapse covers a period of about 20 minutes. Nuclear accumulation of MKL1-GFP is not evident during the course of the video. (MOV 488 kb)
Nuclear translocation of MKL1(1-204)- 2×GFP in Lmna+/+ MEFs
This video shows an Lmna+/+ mouse embryonic fibroblast expressing MKL1(1-204)-2×GFP imaged before (frame 1) and immediately after serum stimulation (frames 2 and onwards). This time lapse covers a period of about 15 minutes. MKL1(1-204)-2×GFP accumulated rapidly in the nucleus during the course of the video. (MOV 64 kb)
Nuclear translocation of MKL1(1-204)- 2×GFP in Lmna–/– MEFs
This video shows an Lmna–/– mouse embryonic fibroblast expressing MKL1(1-204)-2×GFP imaged before (frame 1) and immediately after serum stimulation (frames 2 and onwards). This time lapse covers a period of about 15 minutes. Little or very low levels of MKL1(1-204)-2×GFP accumulated in the nucleus during the course of the video. (MOV 101 kb)
Nuclear translocation of MKL1(1-204)- 2×GFP in Lmna N195K
This video shows an Lmna N195K mouse embryonic fibroblast expressing MKL1(1-204)-2×GFP imaged before (frame 1) and immediately after serum stimulation (frames 2 and onwards). This time lapse covers a period of about 15 minutes. Little or very low levels of MKL1(1-204)-2×GFP accumulated in the nucleus during the course of the video. (MOV 191 kb)
Photoactivation and nuclear translocation of MKL1-PAGFP in Lmna+/+ MEFs
This video shows an Lmna+/+ mouse embryonic fibroblast (outlined in red) expressing MKL1-PAGFP after serum stimulation. Cytoplasmic MKL1-PAGFP was stimulated with a 405 nm laser and entry of the activated pool of MKL1-PAGFP is monitored for 1 minute. Frame 1 was captured before photoactivation. MKL1-PAGFP accumulated in the nucleus during the course of the video. (MOV 528 kb)
Photoactivation and nuclear translocation of MKL1-PAGFP in Lmna–/– MEFs
This video shows an Lmna–/– mouse embryonic fibroblast (outlined in red) expressing MKL1-PAGFP after serum stimulation. Cytoplasmic MKL1-PAGFP was stimulated with a 405 nm laser and entry of the activated pool of MKL1-PAGFP is monitored for 1 minute. Frame 1 was captured before photoactivation. MKL1-PAGFP did not accumulate in the nucleus during the course of the video. (MOV 387 kb)
Photoactivation and nuclear translocation of MKL1-PAGFP in Lmna N195K MEFs
This video shows an Lmna N195K mouse embryonic fibroblast (outlined in red) expressing MKL1-PAGFP after serum stimulation. Cytoplasmic MKL1-PAGFP was stimulated with a 405 nm laser and entry of the activated pool of MKL1-PAGFP is monitored for 1 minute. Frame 1 was captured before photoactivation. MKL1-PAGFP did not accumulate in the nucleus during the course of the video. (MOV 490 kb)
Rights and permissions
About this article
Cite this article
Ho, C., Jaalouk, D., Vartiainen, M. et al. Lamin A/C and emerin regulate MKL1–SRF activity by modulating actin dynamics. Nature 497, 507–511 (2013). https://doi.org/10.1038/nature12105
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12105